Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia
- PMID: 23359706
- PMCID: PMC3574933
- DOI: 10.1073/pnas.1221133110
Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia
Abstract
Mutations in the cytochrome p450 (CYP)21A2 gene, which encodes the enzyme steroid 21-hydroxylase, cause the majority of cases in congenital adrenal hyperplasia, an autosomal recessive disorder. To date, more than 100 CYP21A2 mutations have been reported. These mutations can be associated either with severe salt-wasting or simple virilizing phenotypes or with milder nonclassical phenotypes. Not all CYP21A2 mutations have, however, been characterized biochemically, and the clinical consequences of these mutations remain unknown. Using the crystal structure of its bovine homolog as a template, we have constructed a humanized model of CYP21A2 to provide comprehensive structural explanations for the clinical manifestations caused by each of the known disease-causing missense mutations in CYP21A2. Mutations that affect membrane anchoring, disrupt heme and/or substrate binding, or impair stability of CYP21A2 cause complete loss of function and salt-wasting disease. In contrast, mutations altering the transmembrane region or conserved hydrophobic patches cause up to a 98% reduction in enzyme activity and simple virilizing disease. Mild nonclassical disease can result from interference in oxidoreductase interactions, salt-bridge and hydrogen-bonding networks, and nonconserved hydrophobic clusters. A simple in silico evaluation of previously uncharacterized gene mutations could, thus, potentially help predict the often diverse phenotypes of a monogenic disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
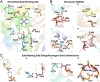
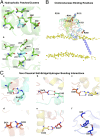

References
-
- New MI. Extensive clinical experience: Nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(11):4205–4214. - PubMed
-
- Wajnrajch MP, New MI. Defects of Adrenal Steroidogenesis. In: Jameson JL, De Groot LJ, editors. Endocrinology. 2010. (Elsevier Philadelphia PA), 6th Ed, Vol 2, pp 1897–1920.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

