Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization
- PMID: 23359810
- PMCID: PMC3554641
- DOI: 10.1371/journal.pone.0054747
Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization
Abstract
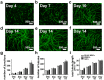
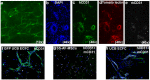
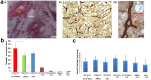
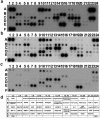
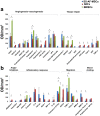
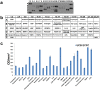
Human amniotic fluid obtained at amniocentesis, when cultured, generates at least two morphologically distinct mesenchymal stem/stromal cell (MSC) subsets. Of these, the spindle shaped amniotic fluid MSCs (SS-AF-MSCs) contain multipotent cells with enhanced adipogenic, osteogenic and chondrogenic capacity. Here, we demonstrate, for the first time, the capacity of these SS-AF-MSCs to support neovascularization by umbilical cord blood (UCB) endothelial colony forming cell (ECFC) derived cells in both in vitro and in vivo models. Interestingly, although the kinetics of vascular tubule formation in vitro were similar when the supporting SS-AF-MSCs were compared with the best vasculogenic supportive batches of bone marrow MSCs (BMSCs) or human dermal fibroblasts (hDFs), SS-AF-MSCs supported vascular tubule formation in vivo more effectively than BMSCs. In NOD/SCID mice, the human vessels inosculated with murine vessels demonstrating their functionality. Proteome profiler array analyses revealed both common and distinct secretion profiles of angiogenic factors by the SS-AF-MSCs as opposed to the hDFs and BMSCs. Thus, SS-AF-MSCs, which are considered to be less mature developmentally than adult BMSCs, and intermediate between adult and embryonic stem cells in their potentiality, have the additional and very interesting potential of supporting increased neovascularisation, further enhancing their promise as vehicles for tissue repair and regeneration.
Conflict of interest statement
Figures

References
-
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV (1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17: 331–340. - PubMed
-
- Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9: 641–650. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. - PubMed
-
- Dexter TM, Allen TD, Lajtha LG (1977) Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol 91: 335–344. - PubMed
-
- English K, French A, Wood KJ (2011) Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell 7: 431–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

