Host defense at the ocular surface
- PMID: 23360155
- PMCID: PMC3750950
- DOI: 10.3109/08830185.2012.749400
Host defense at the ocular surface
Abstract
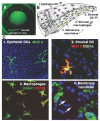
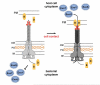
Microbial infections of the cornea frequently cause painful, blinding and debilitating disease that is often difficult to treat and may require corneal transplantation. In addition, sterile corneal infiltrates that are associated with contact lens wear cause pain, visual impairment and photophobia. In this article, we review the role of Toll-Like Receptors (TLR) in bacterial keratitis and sterile corneal infiltrates, and describe the role of MD-2 regulation in LPS responsiveness by corneal epithelial cells. We conclude that both live bacteria and bacterial products activate Toll-Like Receptors in the cornea, which leads to chemokine production and neutrophil recruitment to the corneal stroma. While neutrophils are essential for bacterial killing, they also cause tissue damage that results in loss of corneal clarity. These disparate outcomes, therefore, represent a spectrum of disease severity based on this pathway, and further indicate that targeting the TLR pathway is a feasible approach to treating inflammation caused by live bacteria and microbial products. Further, as the P. aeruginosa type III secretion system (T3SS) also plays a critical role in disease pathogenesis by inducing neutrophil apoptosis and facilitating bacterial growth in the cornea, T3SS exotoxins are additional targets for therapy for P. aeruginosa keratitis.
Figures




References
-
- Saint Andre A, Blackwell NM, Hall LR, et al. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002;295:1892–1895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
