Radiosensitizing effect of intratumoral interleukin-12 gene electrotransfer in murine sarcoma
- PMID: 23360213
- PMCID: PMC3562515
- DOI: 10.1186/1471-2407-13-38
Radiosensitizing effect of intratumoral interleukin-12 gene electrotransfer in murine sarcoma
Abstract
Background: Interleukin-12 (IL-12) based radiosensitization is an effective way of tumor treatment. Local cytokine production, without systemic shedding, might provide clinical benefit in radiation treatment of sarcomas. Therefore, the aim was to stimulate intratumoral IL-12 production by gene electrotransfer of plasmid coding for mouse IL-12 (mIL-12) into the tumors, in order to explore its radiosensitizing effect after single or multiple intratumoral gene electrotransfer.
Methods: Solid SA-1 fibrosarcoma tumors, on the back of A/J mice, were treated intratumorally by mIL-12 gene electrotransfer and 24 h later irradiated with a single dose. Treatment effectiveness was measured by tumor growth delay and local tumor control assay (TCD(50) assay). With respect to therapeutic index, skin reaction in the radiation field was scored. The tumor and serum concentrations of cytokines mIL-12 and mouse interferon γ (mIFNγ) were measured. Besides single, also multiple intratumoral mIL-12 gene electrotransfer before and after tumor irradiation was evaluated.
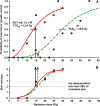
Results: Single intratumoral mIL-12 gene electrotransfer resulted in increased intratumoral but not serum mIL-12 and mIFNγ concentrations, and had good antitumor (7.1% tumor cures) and radiosensitizing effect (21.4% tumor cures). Combined treatment resulted in the radiation dose-modifying factor of 2.16. Multiple mIL-12 gene electrotransfer had an even more pronounced antitumor (50% tumor cures) and radiosensitizing (86.7% tumor cures) effect.
Conclusions: Single or multiple intratumoral mIL-12 gene electrotransfer resulted in increased intratumoral mIL-12 and mIFNγ cytokine level, and may provide an efficient treatment modality for soft tissue sarcoma as single or adjuvant therapy to tumor irradiation.
Figures


Similar articles
-
Potentiation of electrochemotherapy by intramuscular IL-12 gene electrotransfer in murine sarcoma and carcinoma with different immunogenicity.Radiol Oncol. 2012 Dec;46(4):302-11. doi: 10.2478/v10019-012-0044-9. Epub 2012 Nov 9. Radiol Oncol. 2012. PMID: 23412658 Free PMC article.
-
Local and systemic antitumor effect of intratumoral and peritumoral IL-12 electrogene therapy on murine sarcoma.Cancer Biol Ther. 2009 Nov;8(22):2114-22. doi: 10.4161/cbt.8.22.9734. Epub 2009 Nov 5. Cancer Biol Ther. 2009. PMID: 19755854
-
Controlled systemic release of interleukin-12 after gene electrotransfer to muscle for cancer gene therapy alone or in combination with ionizing radiation in murine sarcomas.J Gene Med. 2009 Dec;11(12):1125-37. doi: 10.1002/jgm.1403. J Gene Med. 2009. PMID: 19777440
-
Towards the mechanisms for efficient gene transfer into cells and tissues by means of cell electroporation.Expert Opin Biol Ther. 2012 Mar;12(3):275-86. doi: 10.1517/14712598.2012.654775. Epub 2012 Feb 17. Expert Opin Biol Ther. 2012. PMID: 22339479 Review.
-
Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer.Cancer Immunol Immunother. 2015 Oct;64(10):1315-27. doi: 10.1007/s00262-015-1724-2. Epub 2015 Jun 12. Cancer Immunol Immunother. 2015. PMID: 26067277 Free PMC article. Review.
Cited by
-
Evaluation of p21 promoter for interleukin 12 radiation induced transcriptional targeting in a mouse tumor model.Mol Cancer. 2013 Nov 12;12(1):136. doi: 10.1186/1476-4598-12-136. Mol Cancer. 2013. PMID: 24219565 Free PMC article.
-
A virus based vaccine combined with IL12 gene therapy eradicates aggressive melanoma.Sci Rep. 2025 May 29;15(1):18786. doi: 10.1038/s41598-025-04026-z. Sci Rep. 2025. PMID: 40442309 Free PMC article.
-
Radiotherapy as a New Player in Immuno-Oncology.Cancers (Basel). 2018 Dec 14;10(12):515. doi: 10.3390/cancers10120515. Cancers (Basel). 2018. PMID: 30558196 Free PMC article. Review.
-
Localized Interleukin-12 for Cancer Immunotherapy.Front Immunol. 2020 Oct 15;11:575597. doi: 10.3389/fimmu.2020.575597. eCollection 2020. Front Immunol. 2020. PMID: 33178203 Free PMC article. Review.
-
Electroporation-Based Treatments in Small Animal Veterinary Oral and Maxillofacial Oncology.Front Vet Sci. 2020 Sep 29;7:575911. doi: 10.3389/fvets.2020.575911. eCollection 2020. Front Vet Sci. 2020. PMID: 33134356 Free PMC article. Review.
References
-
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. - PubMed
-
- Kamensek U, Sersa G. Targeted gene therapy in radiotherapy. Radiol Oncol. 2008;42:115–135. doi: 10.2478/v10019-008-0009-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

