Gene-guided gefitinib switch maintenance therapy for patients with advanced EGFR mutation-positive non-small cell lung cancer: an economic analysis
- PMID: 23360224
- PMCID: PMC3568065
- DOI: 10.1186/1471-2407-13-39
Gene-guided gefitinib switch maintenance therapy for patients with advanced EGFR mutation-positive non-small cell lung cancer: an economic analysis
Abstract
Background: Maintenance therapy with gefitinib notably improves survival in patients with advanced non-small cell lung cancer (NSCLC) and EGFR mutation-positive tumors, but the economic impact of this practice is unclear.
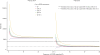
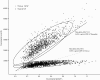
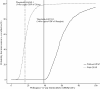
Methods: A decision-analytic model was developed to simulate 21-day patient transitions in a 10-year time horizon. The clinical data were primarily obtained from the results of a pivotal phase III trial that assessed gefitinib maintenance treatment in patients with advanced NSCLC. The cost data were derived from the perspective of the Chinese health care system. The primary outcome was the incremental cost-effectiveness ratio (ICER) at a willingness-to-pay (WTP) threshold of 3 times the per capita GDP of China. Sensitivity analyses were used to explore the impact of uncertainty regarding the results. The impact of the gefitinib patient assistance program (GPAP) was evaluated.
Results: After EGFR genotyping, gefitinib maintenance treatment for advanced NSCLC with EGFR mutations increased the life expectancy by 0.74 years and 0.46 QALYs compared with routine follow-up at an additional cost of $26,149.90 USD ($7,178.20 with the GPAP). The ICER for gefitinib maintenance was $57,066.40 and $15,664.80 per QALY gained (at a 3% discount rate) without and with the GPAP, respectively. The utility of progression free survival, the hazard ratio of progression-free survival for gefitinib treatment and the cost of gefitinib per dose were the three factors that had the greatest influence on the results.
Conclusions: These results indicate that gene-guided maintenance therapy with gefitinib with the GPAP might be a cost-effective treatment option.
Figures


References
-
- Felip E, Stahel RA, Pavlidis N. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of non-small-cell lung cancer (NSCLC) Ann Oncol. 2005;16(Suppl 1):i28–i29. - PubMed
-
- Gridelli C, Ardizzoni A, Douillard JY, Hanna N, Manegold C, Perrone F, Pirker R, Rosell R, Shepherd FA, De Petris L. et al.Recent issues in first-line treatment of advanced non-small-cell lung cancer: results of an international expert panel meeting of the italian association of thoracic oncology. Lung Cancer. 2010;68(3):319–331. doi: 10.1016/j.lungcan.2009.11.018. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

