Altered neuronal density and neurotransmitter expression in the ganglionated region of Ednrb null mice: implications for Hirschsprung's disease
- PMID: 23360229
- PMCID: PMC3578114
- DOI: 10.1111/nmo.12083
Altered neuronal density and neurotransmitter expression in the ganglionated region of Ednrb null mice: implications for Hirschsprung's disease
Abstract
Background: Hirschsprung's disease (HSCR) is a congenital condition in which enteric ganglia, formed from neural crest cells (NCC), are absent from the terminal bowel. Dysmotility and constipation are common features of HSCR that persist following surgical intervention. This persistence suggests that the portion of the colon that remains postoperatively is not able to support normal bowel function. To elucidate the defects that underlie this condition, we utilized a murine model of HSCR.
Methods: Mice with NCC-specific deletion of Ednrb were used to measure the neuronal density and neurotransmitter expression in ganglia.
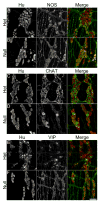
Key results: At the site located proximal to the aganglionic region of P21 Ednrb null mice, the neuronal density is significantly decreased and the expression of neurotransmitters is altered compared with het animals. The ganglia in this colonic region are smaller and more isolated while the size of neuronal cell bodies is increased. The percentage of neurons expressing neuronal nNOS and VIP is significantly increased in Ednrb nulls. Conversely, the percentage of choline acetyltransferase (ChAT) expressing neurons is decreased, while Substance P is unchanged between the two genotypes. These changes are limited to the colon and are not detected in the ileum.
Conclusions & inferences: We demonstrate changes in neuronal density and alterations in the balance of expression of neurotransmitters in the colon proximal to the aganglionic region in Ednrb null mice. The reduced neuronal density and complementary changes in nNOS and ChAT expression may account for the dysmotility seen in HSCR.
© 2013 Blackwell Publishing Ltd.
Conflict of interest statement
No competing interests declared.
Figures







References
-
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, et al. Hirschsprung disease, associated syndromes and genetics: a review. Journal Medical Genetics. 2008;45:1–14. - PubMed
-
- Kenny SE, Tam PK, Garcia-Barcelo M. Hirschsprung’s disease. Semin Pediatr Surg. 2010;19:194–200. - PubMed
-
- Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Current Opinion In Pediatrics. 2000;12:610–7. - PubMed
-
- McCallion AS, Chakravarti A. EDNRB/EDN3 and Hirschsprung disease type II. Pigment Cell Res. 2001;14:161–9. - PubMed
-
- Heanue T, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

