The Bruton tyrosine kinase (BTK) inhibitor PCI-32765 synergistically increases proteasome inhibitor activity in diffuse large-B cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) cells sensitive or resistant to bortezomib
- PMID: 23360303
- PMCID: PMC3739300
- DOI: 10.1111/bjh.12206
The Bruton tyrosine kinase (BTK) inhibitor PCI-32765 synergistically increases proteasome inhibitor activity in diffuse large-B cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) cells sensitive or resistant to bortezomib
Erratum in
-
Corrigendum.Br J Haematol. 2019 Mar;184(5):884-886. doi: 10.1111/bjh.15766. Br J Haematol. 2019. PMID: 30775794 No abstract available.
Abstract
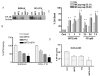
Interactions between the Bruton tyrosine kinase (BTK) inhibitor PCI-32765 and the proteasome inhibitor (bortezomib) were examined in diffuse large-B cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) cells, including those highly resistant to bortezomib. Co-administration of PCI-32765/bortezomib synergistically increased mitochondrial injury and apoptosis in germinal centre- or activated B-cell-like-DLBCL cells and in MCL cells. These events were accompanied by marked AKT and nuclear factor (NF)-κB (NFKB1) inactivation, down-regulation of Mcl-1 (MCL1), Bcl-xL (BCL2L1), and XIAP, and enhanced DNA damage (e.g., γH2A.X formation) and endoplasmic reticulum (ER) stress. Similar interactions were observed in highly bortezomib-resistant DLBCL and MCL cells, and in primary DLBCL cells. In contrast, PCI-32765/bortezomib regimens displayed minimal toxicity toward normal CD34(+) bone marrow cells. Transfection of DLBCL cells with a constitutively active AKT construct attenuated AKT inactivation and significantly diminished cell death, whereas expression of an NF-κB "super-repressor" (IκBαser34/36 ) increased both PCI-32765 and bortezomib lethality. Moreover, cells in which the ER stress response was disabled by a dominant-negative eIF2α construct were resistant to this regimen. Finally, combined exposure to PCI-32765 and bortezomib resulted in more pronounced and sustained reactive oxygen species (ROS) generation, and ROS scavengers significantly diminished lethality. Given promising early clinical results for PCI-32765 in DLBCL and MCL, a strategy combining BTK/proteasome inhibitor warrants attention in these malignancies.
© 2013 Blackwell Publishing Ltd.
Figures
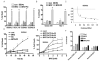
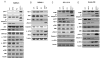

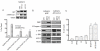


Comment in
-
Findings of Research Misconduct.NIH Guide Grants Contracts (Bethesda). 2015 Dec 18:NOT-OD-16-040. NIH Guide Grants Contracts (Bethesda). 2015. PMID: 26693581 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2015 Dec 10;80(237):76703-76704. Fed Regist. 2015. PMID: 27737268 Free PMC article. No abstract available.
References
-
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. - PubMed
-
- An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death.Differ. 1998;5:1062–1075. - PubMed
-
- Balbasubramanian S, Crowley S, Sirisiwad M, Thiemann P, Chen J, Buggy J. The Bruton’s Tyrosine Kinase (BTK) Inhibitor PCI-32765 Inhibits Growth of ABC DLBCL Tumors In Vivo and in Vitro by Preventing Activation of Pro-Survival NF-{kappa}B pathways. Blood (ASH annual Meeting Abstracts) 2011;117:4969.
-
- Bea S, Zettl A, Wright G, Salaverria I, Jehn P, Moreno V, Burek C, Ott G, Puig X, Yang L, Lopez-Guillermo A, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Gascoyne RD, Connors JM, Grogan TM, Braziel R, Fisher RI, Smeland EB, Kvaloy S, Holte H, Delabie J, Simon R, Powell J, Wilson WH, Jaffe ES, Montserrat E, Muller-Hermelink HK, Staudt LM, Campo E, Rosenwald A. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood. 2005;106:3183–3190. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50CA142509/CA/NCI NIH HHS/United States
- CA63753/CA/NCI NIH HHS/United States
- R01 CA100866/CA/NCI NIH HHS/United States
- R01 CA063753/CA/NCI NIH HHS/United States
- R01 CA093738/CA/NCI NIH HHS/United States
- R01 CA167708/CA/NCI NIH HHS/United States
- CA100866/CA/NCI NIH HHS/United States
- P50 CA130805/CA/NCI NIH HHS/United States
- R01 CA141703/CA/NCI NIH HHS/United States
- CA93738/CA/NCI NIH HHS/United States
- R01 DK052825/DK/NIDDK NIH HHS/United States
- R01 CA150214/CA/NCI NIH HHS/United States
- 1P50 CA130805/CA/NCI NIH HHS/United States
- P50 CA142509/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

