Direct head-to-head comparison of cationic liposome-mediated gene delivery to mesenchymal stem/stromal cells of different human sources: a comprehensive study
- PMID: 23360350
- PMCID: PMC4015075
- DOI: 10.1089/hgtb.2012.185
Direct head-to-head comparison of cationic liposome-mediated gene delivery to mesenchymal stem/stromal cells of different human sources: a comprehensive study
Abstract
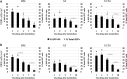
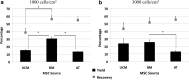
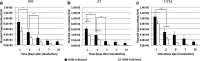
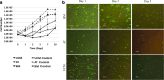
Nonviral gene delivery to human mesenchymal stem/stromal cells (MSC) can be considered a very promising strategy to improve their intrinsic features, amplifying the therapeutic potential of these cells for clinical applications. In this work, we performed a comprehensive comparison of liposome-mediated gene transfer efficiencies to MSC derived from different human sources-bone marrow (BM MSC), adipose tissue-derived cells (ASC), and umbilical cord matrix (UCM MSC). The results obtained using a green fluorescent protein (GFP)-encoding plasmid indicated that MSC isolated from BM and UCM are more amenable to genetic modification when compared to ASC as they exhibited superior levels of viable, GFP(+) cells 48 hr post-transfection, 58 ± 7.1% and 54 ± 3.8%, respectively, versus 33 ± 4.7%. For all cell sources, high cell recoveries (≈50%) and viabilities (>85%) were achieved, and the transgene expression was maintained for 10 days. Levels of plasmid DNA uptake, as well as kinetics of transgene expression and cellular division, were also determined. Importantly, modified cells were found to retain their characteristic immunophenotypic profile and multilineage differentiation capacity. By using the lipofection protocol optimized herein, we were able to maximize transfection efficiencies to human MSC (maximum of 74% total GFP(+) cells) and show that lipofection is a promising transfection strategy for MSC genetic modification, especially when a transient expression of a therapeutic gene is required. Importantly, we also clearly demonstrated that intrinsic features of MSC from different sources should be taken into consideration when developing and optimizing strategies for MSC engineering with a therapeutic gene.
Figures

Similar articles
-
Nonviral gene delivery to mesenchymal stem cells using cationic liposomes for gene and cell therapy.J Biomed Biotechnol. 2010;2010:735349. doi: 10.1155/2010/735349. Epub 2010 Jun 24. J Biomed Biotechnol. 2010. PMID: 20625411 Free PMC article.
-
Engineering of Human Mesenchymal Stem/Stromal Cells with Vascular Endothelial Growth Factor-Encoding Minicircles for Angiogenic Ex Vivo Gene Therapy.Hum Gene Ther. 2019 Mar;30(3):316-329. doi: 10.1089/hum.2018.154. Epub 2018 Nov 13. Hum Gene Ther. 2019. PMID: 30200778
-
Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2017 Apr 26;8(1):102. doi: 10.1186/s13287-017-0555-9. Stem Cell Res Ther. 2017. PMID: 28446235 Free PMC article.
-
Mesenchymal stem cell-based tumor-targeted gene therapy in gastrointestinal cancer.Stem Cells Dev. 2012 Sep 1;21(13):2355-63. doi: 10.1089/scd.2012.0060. Epub 2012 Jun 26. Stem Cells Dev. 2012. PMID: 22530882 Free PMC article. Review.
-
New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity.Stem Cells Dev. 2010 Apr;19(4):423-38. doi: 10.1089/scd.2009.0299. Stem Cells Dev. 2010. PMID: 19958166 Review.
Cited by
-
Non-viral gene delivery to human mesenchymal stem cells: a practical guide towards cell engineering.J Biol Eng. 2023 Jul 25;17(1):49. doi: 10.1186/s13036-023-00363-7. J Biol Eng. 2023. PMID: 37491322 Free PMC article. Review.
-
The study of the intercellular trafficking of the fusion proteins of herpes simplex virus protein VP22.PLoS One. 2014 Jun 23;9(6):e100840. doi: 10.1371/journal.pone.0100840. eCollection 2014. PLoS One. 2014. PMID: 24955582 Free PMC article.
-
Electroporation: A Sustainable and Cell Biology Preserving Cell Labeling Method for Adipogenous Mesenchymal Stem Cells.Biores Open Access. 2019 Mar 29;8(1):32-44. doi: 10.1089/biores.2019.0001. eCollection 2019. Biores Open Access. 2019. PMID: 30944770 Free PMC article.
-
Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness.Stem Cell Rev Rep. 2020 Oct;16(5):812-827. doi: 10.1007/s12015-020-10000-1. Stem Cell Rev Rep. 2020. PMID: 32671645 Free PMC article. Review.
-
Repeated cell sorting ensures the homogeneity of ocular cell populations expressing a transgenic protein.PLoS One. 2022 Mar 25;17(3):e0265183. doi: 10.1371/journal.pone.0265183. eCollection 2022. PLoS One. 2022. PMID: 35333876 Free PMC article.
References
-
- Aluigi M. Fogli M. Curti A, et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow–derived mesenchymal stem cells. Stem Cells. 2006;24:454–461. - PubMed
-
- Aslan H. Zilberman Y. Arbeli V, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889. - PubMed
-
- Azzoni A.R. Ribeiro S.C. Monteiro G.A. Prazeres D.M.F. The impact of polyadenylation signals on plasmid nuclease-resistance and transgene expression. J. Gene Med. 2007;9:392–402. - PubMed
-
- Ben-Ami E. Berrih-Aknin S. Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmunity Reviews. 2011;10:410–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

