miR-296-3p, miR-298-5p and their downstream networks are causally involved in the higher resistance of mammalian pancreatic α cells to cytokine-induced apoptosis as compared to β cells
- PMID: 23360399
- PMCID: PMC3571888
- DOI: 10.1186/1471-2164-14-62
miR-296-3p, miR-298-5p and their downstream networks are causally involved in the higher resistance of mammalian pancreatic α cells to cytokine-induced apoptosis as compared to β cells
Abstract
Background: The molecular bases of mammalian pancreatic α cells higher resistance than β to proinflammatory cytokines are very poorly defined. MicroRNAs are master regulators of cell networks, but only scanty data are available on their transcriptome in these cells and its alterations in diabetes mellitus.
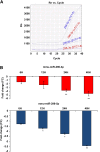
Results: Through high-throughput real-time PCR, we analyzed the steady state microRNA transcriptome of murine pancreatic α (αTC1-6) and β (βTC1) cells: their comparison demonstrated significant differences. We also characterized the alterations of αTC1-6 cells microRNA transcriptome after treatment with proinflammatory cytokines. We focused our study on two microRNAs, miR-296-3p and miR-298-5p, which were: (1) specifically expressed at steady state in αTC1-6, but not in βTC1 or INS-1 cells; (2) significantly downregulated in αTC1-6 cells after treatment with cytokines in comparison to untreated controls. These microRNAs share more targets than expected by chance and were co-expressed in αTC1-6 during a 6-48 h time course treatment with cytokines. The genes encoding them are physically clustered in the murine and human genome. By exploiting specific microRNA mimics, we demonstrated that experimental upregulation of miR-296-3p and miR-298-5p raised the propensity to apoptosis of transfected and cytokine-treated αTC1-6 cells with respect to αTC1-6 cells, treated with cytokines after transfection with scramble molecules. Both microRNAs control the expression of IGF1Rβ, its downstream targets phospho-IRS-1 and phospho-ERK, and TNFα. Our computational analysis suggests that MAFB (a transcription factor exclusively expressed in pancreatic α cells within adult rodent islets of Langerhans) controls the expression of miR-296-3p and miR-298-5p.
Conclusions: Altogether, high-throughput microRNA profiling, functional analysis with synthetic mimics and molecular characterization of modulated pathways strongly suggest that specific downregulation of miR-296-3p and miR-298-5p, coupled to upregulation of their targets as IGF1Rβ and TNFα, is a major determinant of mammalian pancreatic α cells resistance to apoptosis induction by cytokines.
Figures




References
-
- Thomas HE, Graham KL, Chee J, Thomas R, Kay TW, Krishnamurthy B. Proinflammatory cytokines contribute to development and function of regulatory T cells in type 1 diabetes. Ann N Y Acad Sci. in press. - PubMed
-
- Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res Clin Pract. in press. - PubMed
-
- Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53:624–632. doi: 10.2337/diabetes.53.3.624. - DOI - PubMed
-
- Donath MY, Størling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev. 2008;29:334–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

