Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury
- PMID: 23360401
- PMCID: PMC3618589
- DOI: 10.1111/ejn.12119
Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury
Abstract
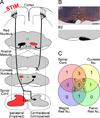
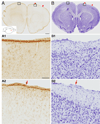
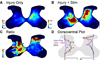
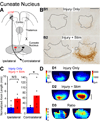
We previously showed that electrical stimulation of motor cortex (M1) after unilateral pyramidotomy in the rat increased corticospinal tract (CST) axon length, strengthened spinal connections, and restored forelimb function. Here, we tested: (i) if M1 stimulation only increases spinal axon length or if it also promotes connections to brain stem forelimb control centers, especially magnocellular red nucleus; and (ii) if stimulation-induced increase in axon length depends on whether pyramidotomy denervated the structure. After unilateral pyramidotomy, we electrically stimulated the forelimb area of intact M1, to activate the intact CST and other corticofugal pathways, for 10 days. We anterogradely labeled stimulated M1 and measured axon length using stereology. Stimulation increased axon length in both the spinal cord and magnocellular red nucleus, even though the spinal cord is denervated by pyramidotomy and the red nucleus is not. Stimulation also promoted outgrowth in the cuneate and parvocellular red nuclei. In the spinal cord, electrical stimulation caused increased axon length ipsilateral, but not contralateral, to stimulation. Thus, stimulation promoted outgrowth preferentially to the sparsely corticospinal-innervated and impaired side. Outgrowth resulted in greater axon density in the ipsilateral dorsal horn and intermediate zone, resembling the contralateral termination pattern. Importantly, as in spinal cord, increase in axon length in brain stem also was preferentially directed towards areas less densely innervated by the stimulated system. Thus, M1 electrical stimulation promotes increases in corticofugal axon length to multiple M1 targets. We propose the axon length change was driven by competition into an adaptive pattern resembling lost connections.
© 2013 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflicts of interest.
Figures





References
-
- Andersen P, Eccles JC, Schmidt RF. Presynaptic inhibition in the cuneate nucleus. Nature. 1962;194:741–743. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
-
- Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. - PubMed
-
- Brown LT. Rubrospinal projections in the rat. J Comp Neurol. 1974;154:169–187. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

