The role of interferon-β in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis - in the perspective of inflammasomes
- PMID: 23360426
- PMCID: PMC3634535
- DOI: 10.1111/imm.12081
The role of interferon-β in the treatment of multiple sclerosis and experimental autoimmune encephalomyelitis - in the perspective of inflammasomes
Abstract
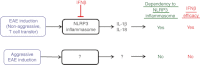
Inflammasomes in innate immune cells mediate the induction of inflammation by sensing microbes and pathogen-associated/damage-associated molecular patterns. Inflammasomes are also known to be involved in the development of some human and animal autoimmune diseases. The Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome is currently the most fully characterized inflammasome, although a limited number of studies have demonstrated its role in demyelinating autoimmune diseases in the central nervous system of humans and animals. Currently, the development of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), is known to be induced by the NLRP3 inflammasome through enhanced recruitment of inflammatory immune cells in the central nervous system. On the other hand, interferon-β (IFNβ), a first-line drug to treat MS, inhibits NLRP3 inflammasome activation, and ameliorates EAE. The NLRP3 inflammasome is indeed a factor capable of inducing EAE, but it is dispensable when EAE is induced by aggressive disease induction regimens. In such NLRP3 inflammasome-independent EAE, IFN-β treatment is generally not effective. This might therefore be one mechanism that leads to occasional failures of IFN-β treatment in EAE, and possibly, in MS as well. In the current review, we discuss inflammasomes and autoimmunity; in particular, the impact of the NLRP3 inflammasome on MS/EAE, and on IFN-β therapy.
© 2013 Blackwell Publishing Ltd.
Figures

References
-
- Kepp O, Galluzzi L, Zitvogel L, Kroemer G. Pyroptosis – a cell death modality of its kind? Eur J Immunol. 2010;40:627–30. - PubMed
-
- Lamkanfi, M Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–20. - PubMed
-
- Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

