Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5
- PMID: 23360514
- PMCID: PMC3609630
- DOI: 10.1089/hum.2012.172
Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5
Abstract
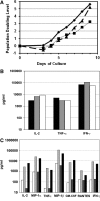
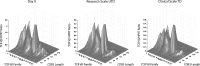
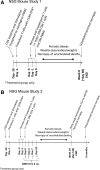
Since HIV requires CD4 and a co-receptor, most commonly C-C chemokine receptor 5 (CCR5), for cellular entry, targeting CCR5 expression is an attractive approach for therapy of HIV infection. Treatment of CD4(+) T cells with zinc-finger protein nucleases (ZFNs) specifically disrupting chemokine receptor CCR5 coding sequences induces resistance to HIV infection in vitro and in vivo. A chimeric Ad5/F35 adenoviral vector encoding CCR5-ZFNs permitted efficient delivery and transient expression following anti-CD3/anti-CD28 costimulation of T lymphocytes. We present data showing CD3/CD28 costimulation substantially improved transduction efficiency over reported methods for Ad5/F35 transduction of T lymphocytes. Modifications to the laboratory scale process, incorporating clinically compatible reagents and methods, resulted in a robust ex vivo manufacturing process capable of generating >10(10) CCR5 gene-edited CD4+ T cells from healthy and HIV+ donors. CD4+ T-cell phenotype, cytokine production, and repertoire were comparable between ZFN-modified and control cells. Following consultation with regulatory authorities, we conducted in vivo toxicity studies that showed no detectable ZFN-specific toxicity or T-cell transformation. Based on these findings, we initiated a clinical trial testing the safety and feasibility of CCR5 gene-edited CD4+ T-cell transfer in study subjects with HIV-1 infection.
Figures



Similar articles
-
Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo.Nat Biotechnol. 2010 Aug;28(8):839-47. doi: 10.1038/nbt.1663. Epub 2010 Jul 2. Nat Biotechnol. 2010. PMID: 20601939 Free PMC article.
-
Optimization of ex vivo activation and expansion of macaque primary CD4-enriched peripheral blood mononuclear cells for use in anti-HIV immunotherapy and gene therapy strategies.J Acquir Immune Defic Syndr. 2003 Mar 1;32(3):245-54. doi: 10.1097/00126334-200303010-00002. J Acquir Immune Defic Syndr. 2003. PMID: 12626883
-
Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases.Nat Biotechnol. 2008 Jul;26(7):808-16. doi: 10.1038/nbt1410. Epub 2008 Jun 29. Nat Biotechnol. 2008. PMID: 18587387 Free PMC article.
-
Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S160-4. doi: 10.1093/infdis/jit382. J Infect Dis. 2013. PMID: 24151324 Free PMC article. Review.
-
Chemokine receptor 5 knockout strategies.Curr Opin HIV AIDS. 2011 Jan;6(1):74-9. doi: 10.1097/COH.0b013e32834122d7. Curr Opin HIV AIDS. 2011. PMID: 21242897 Free PMC article. Review.
Cited by
-
CAR-T cells leave the comfort zone: current and future applications beyond cancer.Immunother Adv. 2020 Nov 25;1(1):ltaa006. doi: 10.1093/immadv/ltaa006. eCollection 2021 Jan. Immunother Adv. 2020. PMID: 36284896 Free PMC article. Review.
-
Development of Lentiviral Vectors Simultaneously Expressing Multiple siRNAs Against CCR5, vif and tat/rev Genes for an HIV-1 Gene Therapy Approach.Mol Ther Nucleic Acids. 2016 Apr 19;5(4):e312. doi: 10.1038/mtna.2016.24. Mol Ther Nucleic Acids. 2016. PMID: 27093170 Free PMC article.
-
Recent developments and clinical studies utilizing engineered zinc finger nuclease technology.Cell Mol Life Sci. 2015 Oct;72(20):3819-30. doi: 10.1007/s00018-015-1956-5. Epub 2015 Jun 19. Cell Mol Life Sci. 2015. PMID: 26089249 Free PMC article. Review.
-
CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection.PLoS One. 2014 Dec 26;9(12):e115987. doi: 10.1371/journal.pone.0115987. eCollection 2014. PLoS One. 2014. PMID: 25541967 Free PMC article.
-
Improved cell-penetrating zinc-finger nuclease proteins for precision genome engineering.Mol Ther Nucleic Acids. 2015 Mar 10;4(3):e232. doi: 10.1038/mtna.2015.6. Mol Ther Nucleic Acids. 2015. PMID: 25756962 Free PMC article.
References
-
- Allers K. Hutter G. Hofmann J., et al. Evidence for the cure of HIV infection by CCR5{Delta}32/{Delta}32 stem cell transplantation. Blood. 2011;117:2791–2799. - PubMed
-
- Carrington M. Dean M. Martin M.P. O'Brien S.J. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum. Mol. Genet. 1999;8:1939–1945. - PubMed
-
- Chen Z. Ahonen M. Hamalainen H., et al. High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J. Immunol. Methods. 2002;260:79–89. - PubMed
-
- Cho H.I. Kim H.J. Oh S.T. Kim T.G. In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine. 2003;22:224–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

