Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5
- PMID: 23360514
- PMCID: PMC3609630
- DOI: 10.1089/hum.2012.172
Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5
Abstract
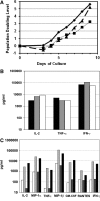
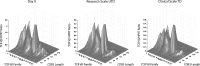
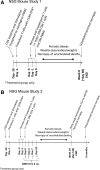
Since HIV requires CD4 and a co-receptor, most commonly C-C chemokine receptor 5 (CCR5), for cellular entry, targeting CCR5 expression is an attractive approach for therapy of HIV infection. Treatment of CD4(+) T cells with zinc-finger protein nucleases (ZFNs) specifically disrupting chemokine receptor CCR5 coding sequences induces resistance to HIV infection in vitro and in vivo. A chimeric Ad5/F35 adenoviral vector encoding CCR5-ZFNs permitted efficient delivery and transient expression following anti-CD3/anti-CD28 costimulation of T lymphocytes. We present data showing CD3/CD28 costimulation substantially improved transduction efficiency over reported methods for Ad5/F35 transduction of T lymphocytes. Modifications to the laboratory scale process, incorporating clinically compatible reagents and methods, resulted in a robust ex vivo manufacturing process capable of generating >10(10) CCR5 gene-edited CD4+ T cells from healthy and HIV+ donors. CD4+ T-cell phenotype, cytokine production, and repertoire were comparable between ZFN-modified and control cells. Following consultation with regulatory authorities, we conducted in vivo toxicity studies that showed no detectable ZFN-specific toxicity or T-cell transformation. Based on these findings, we initiated a clinical trial testing the safety and feasibility of CCR5 gene-edited CD4+ T-cell transfer in study subjects with HIV-1 infection.
Figures



References
-
- Allers K. Hutter G. Hofmann J., et al. Evidence for the cure of HIV infection by CCR5{Delta}32/{Delta}32 stem cell transplantation. Blood. 2011;117:2791–2799. - PubMed
-
- Carrington M. Dean M. Martin M.P. O'Brien S.J. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum. Mol. Genet. 1999;8:1939–1945. - PubMed
-
- Chen Z. Ahonen M. Hamalainen H., et al. High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J. Immunol. Methods. 2002;260:79–89. - PubMed
-
- Cho H.I. Kim H.J. Oh S.T. Kim T.G. In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine. 2003;22:224–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

