High efficacy of CpG-ODN, cetuximab and cisplatin combination for very advanced ovarian xenograft tumors
- PMID: 23360557
- PMCID: PMC3571944
- DOI: 10.1186/1479-5876-11-25
High efficacy of CpG-ODN, cetuximab and cisplatin combination for very advanced ovarian xenograft tumors
Abstract
Background: To mimic clinical treatment situations in advanced human ovarian disease, we tested the efficacy of CpG-oligodeoxynucleotides (CpG-ODN), synthetic DNA sequences recognized by Toll-like receptor 9 and able to induce innate/adaptive immune responses, in combination with other possible therapeutic reagents in ovarian carcinoma ascites-bearing athymic mice.
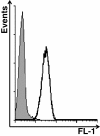
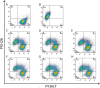
Methods: Mice injected i.p. with IGROV-1 ovarian cancer cells were treated at different stages of ascites progression for 4 weeks with CpG-ODN, alone or in combination with Bevacizumab, Polyinosinic:Polycytidylic acid (Poly(I):Poly(C)), Gefitinib, Cetuximab and Cisplatin. Median survival time (MST) was calculated for each group. IGROV-1 cells treated or not with Cetuximab were assayed for antibody-dependent cellular cytotoxicity by 51Cr-release assay, and for macrophage antibody-dependent cell-mediated phagocytosis by flow cytometry.
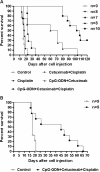
Results: In mice treated when ascitic fluid began to accumulate, CpG-ODN combined with Bevacizumab, Poly(I):Poly(C) or Gefitinib did not significantly increase MST as compared with that using CpG-ODN alone, whereas MST in mice treated with CpG-ODN plus Cetuximab was significantly increased (>103 days for combination vs 62 days for CpG alone; P = 0.0008), with 4/8 mice alive at the end of the experiment. In experiments in mice showing increased abdominal volume and body weight (27.9 ± 0.8 g after vs 23 ± 1.1 g before tumor cell injection), treatment with Cisplatin in addition to CpG-ODN/Cetuximab led to significantly increased MST (105.5 days; P = 0.001), with all mice still alive at 85 days, over that using CpG-ODN/Cetuximab (66 days), Cetuximab/Cisplatin (18.5 days), Cisplatin (23 days) or saline (16 days). At a very advanced stage of disease (body weight: 31.4 ± 0.9 g), when more than half of control mice had to be sacrificed 6 days after starting treatments, the triple-combination therapy still increased MST (45 days; P = 0.0089) vs controls.
Conclusions: CpG-ODN combination therapies that enhance the immune response in the tumor microenvironment and concomitantly target tumor cells are highly efficacious even in experimental advanced malignancies. Although differences in the distribution of TLR9 in mice and humans and the enrichment of this receptor on innate immune cells of athymic mice must be considered, our results indicate a promising strategy to treat ovarian cancer patients with bulky ascites.
Figures

Similar articles
-
Ascites regression and survival increase in mice bearing advanced-stage human ovarian carcinomas and repeatedly treated intraperitoneally with CpG-ODN.J Immunother. 2010 Jan;33(1):8-15. doi: 10.1097/CJI.0b013e3181affaa7. J Immunother. 2010. PMID: 19952960
-
G3139 and other CpG-containing immunostimulatory phosphorothioate oligodeoxynucleotides are potent suppressors of the growth of human tumor xenografts in nude mice.Oligonucleotides. 2006 Spring;16(1):83-93. doi: 10.1089/oli.2006.16.83. Oligonucleotides. 2006. PMID: 16584297
-
TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects.Cancer Res. 2011 Oct 15;71(20):6382-90. doi: 10.1158/0008-5472.CAN-11-1285. Epub 2011 Aug 30. Cancer Res. 2011. PMID: 21878529
-
Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs.Curr Opin Microbiol. 2003 Oct;6(5):472-7. doi: 10.1016/j.mib.2003.09.007. Curr Opin Microbiol. 2003. PMID: 14572539 Review.
-
Synergism between cytosine-guanine oligodeoxynucleotides and monoclonal antibody in the treatment of lymphoma.Semin Oncol. 2002 Feb;29(1 Suppl 2):93-7. Semin Oncol. 2002. PMID: 11842395 Review.
Cited by
-
Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes.Front Immunol. 2021 May 7;12:642285. doi: 10.3389/fimmu.2021.642285. eCollection 2021. Front Immunol. 2021. PMID: 34025653 Free PMC article. Review.
-
CpG oligodeoxynucleotides potentiate the antitumor activity of anti-BST2 antibody.Cancer Sci. 2015 Oct;106(10):1474-8. doi: 10.1111/cas.12738. Cancer Sci. 2015. PMID: 26498112 Free PMC article.
-
Metallic Nanoparticles for Cancer Immunotherapy.Mater Today (Kidlington). 2018 Jul-Aug;21(6):673-685. doi: 10.1016/j.mattod.2017.11.022. Epub 2017 Dec 14. Mater Today (Kidlington). 2018. PMID: 30197553 Free PMC article.
-
Intraperitoneal CMP-001: A Novel Immunotherapy for Treating Peritoneal Carcinomatosis of Gastrointestinal and Pancreaticobiliary Cancer.Ann Surg Oncol. 2021 Feb;28(2):1187-1197. doi: 10.1245/s10434-020-08591-7. Epub 2020 May 14. Ann Surg Oncol. 2021. PMID: 32409965 Free PMC article.
-
CpG-oligodeoxynucleotides exert remarkable antitumor activity against diffuse malignant peritoneal mesothelioma orthotopic xenografts.J Transl Med. 2016 Jan 25;14:25. doi: 10.1186/s12967-016-0781-4. J Transl Med. 2016. PMID: 26810896 Free PMC article.
References
-
- Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26(3–4):737–747. - PubMed
-
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. - PubMed
-
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. - PubMed
-
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. - PubMed
-
- Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–5432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

