Comparison of the therapeutic effects of human and mouse adipose-derived stem cells in a murine model of lipopolysaccharide-induced acute lung injury
- PMID: 23360775
- PMCID: PMC3706907
- DOI: 10.1186/scrt161
Comparison of the therapeutic effects of human and mouse adipose-derived stem cells in a murine model of lipopolysaccharide-induced acute lung injury
Abstract
Introduction: Adipose-derived stem cells (ASCs) have emerged as important regulators of inflammatory/immune responses in vitro and in vivo and represent attractive candidates for cell-based therapies for diseases that involve excessive inflammation. Acute lung injury (ALI) is an inflammatory condition for which treatment is mainly supportive due to lack of effective therapies. In this study, the therapeutic effects of ASC-based therapy were assessed in vivo by comparison of the anti-inflammatory properties of both human and murine ASCs in a mouse model of lipopolysaccharide (LPS)-induced ALI.
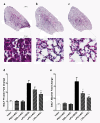
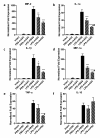
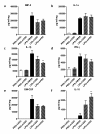
Methods: Human ASCs (hASCs) or mouse ASCs (mASCs) were delivered to C57Bl/6 mice (7.5 × 105 total cells/mouse) by oropharyngeal aspiration (OA) four hours after the animals were challenged with lipopolysaccharide (15 mg/kg). Mice were sacrificed 24 and 72 hours after LPS exposure, and lung histology examined for evaluation of inflammation and injury. Bronchoalveolar lavage fluid (BALF) was analyzed to determine total and differential cell counts, total protein and albumin concentrations, and myeloperoxidase (MPO) activity. Cytokine expression in the injured lungs was measured at the steady-state mRNA levels and protein levels for assessment of the degree of lung inflammation.
Results: Both human and mouse ASC treatments provided protective anti-inflammatory responses. There were decreased levels of leukocyte (for example neutrophil) migration into the alveoli, total protein and albumin concentrations in BALF, and MPO activity after the induction of ALI following both therapies. Additionally, cell therapy with both cell types effectively suppressed the expression of proinflammatory cytokines and increased the anti-inflammatory cytokine interleukin 10 (IL-10). Overall, the syngeneic mASC therapy had a more potent therapeutic effect than the xenogeneic hASC therapy in this model.
Conclusions: Treatment with hASCs or mASCs significantly attenuated LPS-induced acute lung injury in mice. These results suggest a potential benefit for using an ASC-based therapy to treat clinical ALI and may possibly prevent the development of acute respiratory distress syndrome (ARDS).
Figures
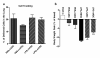

Similar articles
-
Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury.Stem Cells. 2014 Jun;32(6):1616-28. doi: 10.1002/stem.1632. Stem Cells. 2014. PMID: 24449042 Free PMC article.
-
Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury.Am J Respir Cell Mol Biol. 2013 Oct;49(4):552-62. doi: 10.1165/rcmb.2012-0406OC. Am J Respir Cell Mol Biol. 2013. PMID: 23656573
-
Inhibition of Pendrin by a small molecule reduces Lipopolysaccharide-induced acute Lung Injury.Theranostics. 2020 Aug 7;10(22):9913-9922. doi: 10.7150/thno.46417. eCollection 2020. Theranostics. 2020. PMID: 32929324 Free PMC article.
-
[The effect of recombinant interleukin-10/Fc fusion protein on lipopolysaccharide-induced acute lung injury in mice].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008 Aug;20(8):461-4. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008. PMID: 18687171 Chinese.
-
Effects of Obesity on Pulmonary Inflammation and Remodeling in Experimental Moderate Acute Lung Injury.Front Immunol. 2019 May 29;10:1215. doi: 10.3389/fimmu.2019.01215. eCollection 2019. Front Immunol. 2019. PMID: 31275296 Free PMC article.
Cited by
-
Effects of intravenous administration of peripheral blood-derived mesenchymal stromal cells after infusion of lipopolysaccharide in horses.J Vet Intern Med. 2022 Jul;36(4):1491-1501. doi: 10.1111/jvim.16447. Epub 2022 Jun 14. J Vet Intern Med. 2022. PMID: 35698909 Free PMC article.
-
PGE2 Produced by Exogenous MSCs Promotes Immunoregulation in ARDS Induced by Highly Pathogenic Influenza A through Activation of the Wnt-β-Catenin Signaling Pathway.Int J Mol Sci. 2023 Apr 14;24(8):7299. doi: 10.3390/ijms24087299. Int J Mol Sci. 2023. PMID: 37108459 Free PMC article. Review.
-
Conditioned media from adipose stromal cells limit lipopolysaccharide-induced lung injury, endothelial hyperpermeability and apoptosis.J Transl Med. 2015 Feb 21;13:67. doi: 10.1186/s12967-015-0422-3. J Transl Med. 2015. PMID: 25889857 Free PMC article.
-
Reactive Oxygen Species in Mesenchymal Stem Cell Aging: Implication to Lung Diseases.Oxid Med Cell Longev. 2015;2015:486263. doi: 10.1155/2015/486263. Epub 2015 Jul 26. Oxid Med Cell Longev. 2015. PMID: 26273422 Free PMC article. Review.
-
Adipose-derived Mesenchymal Stromal Cells Modulate Lipid Metabolism and Lipid Droplet Biogenesis via AKT/mTOR -PPARγ Signalling in Macrophages.Sci Rep. 2019 Dec 30;9(1):20304. doi: 10.1038/s41598-019-56835-8. Sci Rep. 2019. PMID: 31889120 Free PMC article.
References
-
- Bonvillain RW, Zhang S, Eagle ME, Danchuk SD, Bunnell BA, Sullivan DE. Battling inflammation in acute lung injury and acute respiratory distress syndrome: stem cell-based therapy targeting the root cause of acute lung injury. J Pulmonar Respirat Med. in press .
-
- Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, Gattas DJ, Hallett D, Tomlinson G, Stewart TE, Ferguson ND. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Resp Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

