Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model
- PMID: 23360790
- PMCID: PMC3672720
- DOI: 10.1186/ar4156
Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model
Abstract
Introduction: Cell therapy is a rapidly growing area of research for the treatment of osteoarthritis (OA). This work is aimed to investigate the efficacy of intra-articular adipose-derived stromal cell (ASC) injection in the healing process on cartilage, synovial membrane and menisci in an experimental rabbit model.
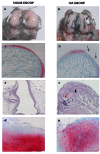
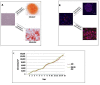
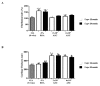
Methods: The induction of OA was performed surgically through bilateral anterior cruciate ligament transection (ACLT) to achieve eight weeks from ACLT a mild grade of OA. A total of 2×10⁶ and 6×10⁶ autologous ASCs isolated from inguinal fat, expanded in vitro and suspended in 4% rabbit serum albumin (RSA) were delivered in the hind limbs; 4% RSA was used as the control. Local bio-distribution of the cells was verified by injecting chloro-methyl-benzamido-1,1'-dioctadecyl-3,3,3'3'-tetra-methyl-indo-carbocyanine per-chlorate (CM-Dil) labeled ASCs in the hind limbs. Cartilage and synovial histological sections were scored by Laverty's scoring system to assess the severity of the pathology. Protein expression of some extracellular matrix molecules (collagen I and II), catabolic (metalloproteinase-1 and -3) and inflammatory (tumor necrosis factor- α) markers were detected by immunohistochemistry. Assessments were carried out at 16 and 24 weeks.
Results: Labeled-ASCs were detected unexpectedly in the synovial membrane and medial meniscus but not in cartilage tissue at 3 and 20 days from ASC-treatment. Intra-articular ASC administration decreases OA progression and exerts a healing contribution in the treated animals in comparison to OA and 4% RSA groups.
Conclusions: Our data reveal a healing capacity of ASCs in promoting cartilage and menisci repair and attenuating inflammatory events in synovial membrane inhibiting OA progression. On the basis of the local bio-distribution findings, the benefits obtained by ASC treatment could be due to a trophic mechanism of action by the release of growth factors and cytokines.
Figures




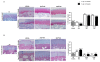



References
-
- Scott JC, Lethbridge-Cejku M, Hochberg MC. In: Osteoarthritis: Clinical and Experimental Aspects. 1. Reginster JY, Pelletier JP, Marte-Pelletier J, Henrotin Y, editor. Vol. 1. Munich, Germany: Springer; 1999. Epidemiology and economic consequences of osteoarthritis; pp. 20–52.
-
- Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66:680–690. - PubMed
-
- Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427(suppl):S27–S36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

