MEK-1 activates C-Raf through a Ras-independent mechanism
- PMID: 23360980
- PMCID: PMC3608709
- DOI: 10.1016/j.bbamcr.2013.01.015
MEK-1 activates C-Raf through a Ras-independent mechanism
Abstract
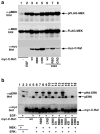
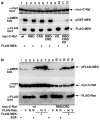
C-Raf is a member of the Ras-Raf-MEK-ERK mitogen-activated protein kinase (MAPK) signaling pathway that plays key roles in diverse physiological processes and is upregulated in many human cancers. C-Raf activation involves binding to Ras, increased phosphorylation and interactions with co-factors. Here, we describe a Ras-independent in vivo pathway for C-Raf activation by its downstream target MEK. Using (32)P-metabolic labeling and 2D-phosphopeptide mapping experiments, we show that MEK increases C-Raf phosphorylation by up-to 10-fold. This increase was associated with C-Raf kinase activation, matching the activity seen with growth factor stimulation. Consequently, coexpression of wildtype C-Raf and MEK was sufficient for full and constitutive activation of ERK. Notably, the ability of MEK to activate C-Raf was completely Ras independent, since mutants impaired in Ras binding that are irresponsive to growth factors or Ras were fully activated by MEK. The ability of MEK to activate C-Raf was only partially dependent on MEK kinase activity but required MEK binding to C-Raf, suggesting that the binding results in a conformational change that increases C-Raf susceptibility to phosphorylation and activation or in the stabilization of the phosphorylated-active form. These findings propose a novel Ras-independent mechanism for activating the C-Raf and the MAPK pathway without the need for mutations in the pathway. This mechanism could be of significance in pathological conditions or cancers overexpressing C-Raf and MEK or in conditions where C-Raf-MEK interaction is enhanced due to the down-regulation of RKIP and MST2.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

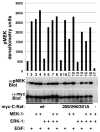
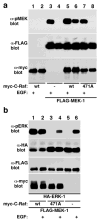
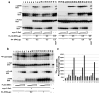


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

