Advanced neuroimaging in traumatic brain injury
- PMID: 23361483
- PMCID: PMC3779469
- DOI: 10.1055/s-0032-1331810
Advanced neuroimaging in traumatic brain injury
Abstract
Advances in structural and functional neuroimaging have occurred at a rapid pace over the past two decades. Novel techniques for measuring cerebral blood flow, metabolism, white matter connectivity, and neural network activation have great potential to improve the accuracy of diagnosis and prognosis for patients with traumatic brain injury (TBI), while also providing biomarkers to guide the development of new therapies. Several of these advanced imaging modalities are currently being implemented into clinical practice, whereas others require further development and validation. Ultimately, for advanced neuroimaging techniques to reach their full potential and improve clinical care for the many civilians and military personnel affected by TBI, it is critical for clinicians to understand the applications and methodological limitations of each technique. In this review, we examine recent advances in structural and functional neuroimaging and the potential applications of these techniques to the clinical care of patients with TBI. We also discuss pitfalls and confounders that should be considered when interpreting data from each technique. Finally, given the vast amounts of advanced imaging data that will soon be available to clinicians, we discuss strategies for optimizing data integration, visualization, and interpretation.
Thieme Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
Figures
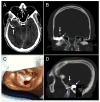
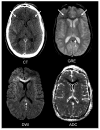
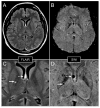
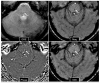
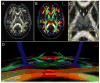
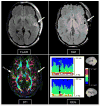

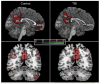
References
-
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
-
- Tanielian TL, Jaycox L Rand Corporation. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND; 2008. p. xliii.p. 453.
-
- Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain. 1974;97(4):633–654. - PubMed
-
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol. 2002;67(4):281–344. - PubMed
-
- Ropper AH, Gorson KC. Clinical practice. Concussion N Engl J Med. 2007;356(2):166–172. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

