Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study
- PMID: 23361612
- PMCID: PMC3968942
- DOI: 10.1176/appi.ajp.2012.12030385
Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study
Abstract
Objective: To investigate the neural substrate of premenstrual dysphoric disorder (PMDD), the authors used [15O]H2O positron emission tomography (PET) regional cerebral blood flow (rCBF) and blood-oxygen-level-dependent (BOLD) functional MRI (fMRI) signal measurements during working memory in conjunction with a 6-month hormone manipulation protocol.
Method: PET and fMRI scans were obtained from women with prospectively confirmed PMDD and asymptomatic comparison subjects while they completed the n-back task during three hormone conditions: ovarian suppression induced by the gonadotropin-releasing hormone agonist leuprolide acetate, leuprolide plus estradiol, and leuprolide plus progesterone. Fifteen patients and 15 matched comparison subjects underwent PET imaging. Fourteen patients and 14 comparison subjects underwent fMRI. For each hormone condition, rCBF was measured with [15O]H2O PET, and BOLD signal was measured with fMRI, both during an n-back working memory paradigm. Global Assessment of Functioning Scale (GAF) scores and clinical characteristics were obtained for each patient before hormone manipulation, and symptoms were measured before and during the protocol.
Results: In both the PET and fMRI studies, a main effect of diagnosis was observed, with PMDD patients showing greater prefrontal activation than comparison subjects. In the patient group, the degree to which dorsolateral prefrontal cortex activation was abnormally increased correlated with several dimensions of disease: disability as indicated by GAF scores, age at symptom onset, duration of PMDD, and differences in pre- and postmenses PMDD symptoms.
Conclusions: Abnormal working memory activation in PMDD, specifically in the dorsolateral prefrontal cortex, is related to PMDD severity, symptoms, age at onset, and disease burden. These results support the clinical relevance of the findings and the proposal that dorsolateral prefrontal cortex dysfunction represents a substrate of risk for PMDD. The concordance of the fMRI and PET data attests to the neurobiological validity of the results.
Conflict of interest statement
The authors report no financial relationships with commercial interests.
Figures


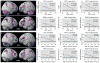
Comment in
-
Premenstrual dysphoric disorder and the brain.Am J Psychiatry. 2013 Mar;170(3):248-52. doi: 10.1176/appi.ajp.2012.12121555. Am J Psychiatry. 2013. PMID: 23450284 Free PMC article. No abstract available.
References
-
- Borenstein J, Chiou CF, Dean B, Wong J, Wade S. Estimating direct and indirect costs of premenstrual syndrome. J Occup Environ Med. 2005;47:26–33. - PubMed
-
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28(suppl 3):1–23. - PubMed
-
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol. 1988;158:5–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

