Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis
- PMID: 23362252
- PMCID: PMC3610957
- DOI: 10.1074/jbc.M112.440503
Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis
Abstract
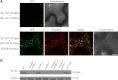
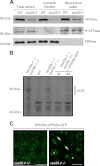
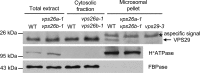
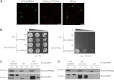
The retromer complex localizes to endosomal membranes and is involved in protein trafficking. In mammals, it is composed of a dimer of sorting nexins and of the core retromer consisting of vacuolar protein sorting (VPS)26, VPS29, and VPS35. Although homologs of these proteins have been identified in plants, how the plant retromer functions remains elusive. To better understand the role of VPS components in the assembly and function of the core retromer, we characterize here Arabidopsis vps26-null mutants. We show that impaired VPS26 function has a dramatic effect on VPS35 levels and causes severe phenotypic defects similar to those observed in vps29-null mutants. This implies that functions of plant VPS26, VPS29, and VPS35 are tightly linked. Then, by combining live-cell imaging with immunochemical and genetic approaches, we report that VPS35 alone is able to bind to endosomal membranes and plays an essential role in VPS26 and VPS29 membrane recruitment. We also show that the Arabidopsis Rab7 homolog RABG3f participates in the recruitment of the core retromer to the endosomal membrane by interacting with VPS35. Altogether our data provide original information on the molecular interactions that mediate assembly of the core retromer in plants.
Figures




References
-
- Attar N., Cullen P. J. (2010) The retromer complex. Adv. Enzyme Regul. 50, 216–236 - PubMed
-
- Wassmer T., Attar N., Bujny M. V., Oakley J., Traer C. J., Cullen P. J. (2007) A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J. Cell Sci. 120, 45–54 - PubMed
-
- Wassmer T., Attar N., Harterink M., van Weering J. R., Traer C. J., Oakley J., Goud B., Stephens D. J., Verkade P., Korswagen H. C., Cullen P. J. (2009) The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev. Cell 17, 110–122 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

