Thioredoxin-related protein 32 (TRP32) specifically reduces oxidized phosphatase of regenerating liver (PRL)
- PMID: 23362275
- PMCID: PMC3591634
- DOI: 10.1074/jbc.M112.418004
Thioredoxin-related protein 32 (TRP32) specifically reduces oxidized phosphatase of regenerating liver (PRL)
Abstract
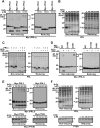
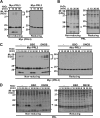
PRL family constitutes a unique class of phosphatases associated with metastasis. The phosphatase activity of PRL has been reported to be important for promoting metastasis, and it is inactivated by reversible oxidation of its catalytic cysteine. Here, we show that TRP32 specifically reduces PRL. Reduction of oxidized PRL in cells is inhibited by 2,4-dinitro-1-chlorobenzene, an inhibitor of TRX reductase. In vitro assays for the reduction of PRL show that only TRP32 can potently reduce oxidized PRL, whereas other TRX-related proteins linked to TRX reductase show little or no reducing activity. Indeed, TRP32 knockdown significantly prolongs the H2O2-induced oxidation of PRL. Binding analyses reveal that the unique C-terminal domain of TRP32 is required and sufficient for its direct interaction with PRL. These results suggest that TRP32 maintains the reduced state of PRL and thus regulates the biological function of PRL.
Figures



Similar articles
-
Oxidative stress-induced expression and modulation of Phosphatase of Regenerating Liver-1 (PRL-1) in mammalian retina.Biochim Biophys Acta. 2007 Sep;1773(9):1473-82. doi: 10.1016/j.bbamcr.2007.06.005. Epub 2007 Jun 26. Biochim Biophys Acta. 2007. PMID: 17673310 Free PMC article.
-
Enzyme activity of phosphatase of regenerating liver is controlled by the redox environment and its C-terminal residues.Biochemistry. 2009 May 26;48(20):4262-72. doi: 10.1021/bi900241k. Biochemistry. 2009. PMID: 19341304 Free PMC article.
-
Molecular mechanisms of the PRL phosphatases.FEBS J. 2013 Jan;280(2):505-24. doi: 10.1111/j.1742-4658.2012.08565.x. Epub 2012 Apr 10. FEBS J. 2013. PMID: 22413991 Review.
-
PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility.Cancer Res. 2006 Mar 15;66(6):3153-61. doi: 10.1158/0008-5472.CAN-05-3116. Cancer Res. 2006. PMID: 16540666
-
Physiological and oncogenic roles of the PRL phosphatases.FEBS J. 2018 Nov;285(21):3886-3908. doi: 10.1111/febs.14503. Epub 2018 May 27. FEBS J. 2018. PMID: 29770564 Review.
Cited by
-
Tat-Thioredoxin-like protein 1 attenuates ischemic brain injury by regulation of MAPKs and apoptosis signaling.BMB Rep. 2023 Apr;56(4):234-239. doi: 10.5483/BMBRep.2022-0184. BMB Rep. 2023. PMID: 36571143 Free PMC article.
-
Development of a high-throughput screening system targeting the protein-protein interactions between PRL and CNNM.Sci Rep. 2024 Oct 25;14(1):25432. doi: 10.1038/s41598-024-76269-1. Sci Rep. 2024. PMID: 39455715 Free PMC article.
-
Multifunctional Thioredoxin-Like Protein from the Gastrointestinal Parasitic Nematodes Strongyloides ratti and Trichuris suis Affects Mucosal Homeostasis.J Parasitol Res. 2016;2016:8421597. doi: 10.1155/2016/8421597. Epub 2016 Oct 31. J Parasitol Res. 2016. PMID: 27872753 Free PMC article.
-
TXNL1 has dual functions as a redox active thioredoxin-like protein as well as an ATP- and redox-independent chaperone.Redox Biol. 2023 Nov;67:102897. doi: 10.1016/j.redox.2023.102897. Epub 2023 Sep 26. Redox Biol. 2023. PMID: 37804695 Free PMC article.
-
Next-Generation Cell-Active Inhibitors of the Undrugged Oncogenic PTP4A3 Phosphatase.J Pharmacol Exp Ther. 2019 Dec;371(3):652-662. doi: 10.1124/jpet.119.262188. Epub 2019 Oct 10. J Pharmacol Exp Ther. 2019. PMID: 31601683 Free PMC article.
References
-
- Cates C. A., Michael R. L., Stayrook K. R., Harvey K. A., Burke Y. D., Randall S. K., Crowell P. L., Crowell D. N. (1996) Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 110, 49–55 - PubMed
-
- Rios P., Li X., Köhn M. (2013) Molecular mechanisms of the PRL phosphatases. FEBS J. 280, 505–524 - PubMed
-
- Bessette D. C., Qiu D., Pallen C. J. (2008) PRL PTPs: mediators and markers of cancer progression. Cancer Metastasis Rev. 27, 231–252 - PubMed
-
- Zeng Q., Dong J. M., Guo K., Li J., Tan H. X., Koh V., Pallen C. J., Manser E., Hong W. (2003) PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res. 63, 2716–2722 - PubMed
-
- Al-Aidaroos A. Q., Zeng Q. (2010) PRL-3 phosphatase and cancer metastasis. J. Cell Biochem. 111, 1087–1098 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

