Dentin phosphophoryn activates Smad protein signaling through Ca2+-calmodulin-dependent protein kinase II in undifferentiated mesenchymal cells
- PMID: 23362283
- PMCID: PMC3605677
- DOI: 10.1074/jbc.M112.413997
Dentin phosphophoryn activates Smad protein signaling through Ca2+-calmodulin-dependent protein kinase II in undifferentiated mesenchymal cells
Abstract
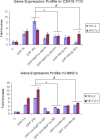
Dentin phosphophoryn (DPP) is a major noncollagenous protein in the dentin matrix. In this study, we demonstrate that pluripotent stem cells such as C3H10T1/2 and human bone marrow cells can be committed to the osteogenic lineage by DPP. Treatment with DPP can stimulate the release of intracellular Ca(2+). This calcium flux triggered the activation of Ca(2+)-calmodulin-dependent protein kinase II (CaMKII). Activated CaMKII induced the phosphorylation of Smad1 and promoted nuclear translocation of p-Smad1. Inhibition of store Ca(2+) depletion by 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester) or down-regulation of CaMKII by KN-62, a selective cell-permeable pharmacological inhibitor or a dominant negative plasmid of CaMKII, blocked DPP-mediated Smad1 phosphorylation. Activation of Smad1 resulted in the expression of osteogenic markers such as Runx2, Osterix, DMP1, Bone sialoprotein, Osteocalcin, NFATc1, and Schnurri-2, which have been implicated in osteoblast differentiation. These findings suggest that DPP is capable of triggering commitment of pluripotent stem cells to the osteogenic lineage.
Figures







Similar articles
-
A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells.Cell Signal. 2008 Apr;20(4):613-24. doi: 10.1016/j.cellsig.2007.11.012. Epub 2007 Nov 29. Cell Signal. 2008. PMID: 18248957
-
Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling.J Biol Chem. 2016 Jan 15;291(3):1148-61. doi: 10.1074/jbc.M115.668939. Epub 2015 Oct 15. J Biol Chem. 2016. PMID: 26472929 Free PMC article.
-
Extracellular matrix-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of bone morphogenetic protein.J Biol Chem. 2006 Mar 3;281(9):5341-7. doi: 10.1074/jbc.M506158200. Epub 2005 Dec 2. J Biol Chem. 2006. PMID: 16326713
-
Dentin phosphoprotein (DPP) activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells.J Biol Chem. 2012 Feb 17;287(8):5211-24. doi: 10.1074/jbc.M111.290080. Epub 2011 Dec 1. J Biol Chem. 2012. PMID: 22134916 Free PMC article.
-
The nature and functional significance of dentin extracellular matrix proteins.Int J Dev Biol. 1995 Feb;39(1):169-79. Int J Dev Biol. 1995. PMID: 7626404 Review.
Cited by
-
Dentine sialophosphoprotein signal in dentineogenesis and dentine regeneration.Eur Cell Mater. 2021 Jul 18;42:43-62. doi: 10.22203/eCM.v042a04. Eur Cell Mater. 2021. PMID: 34275129 Free PMC article. Review.
-
DPP promotes odontogenic differentiation of DPSCs through NF-κB signaling.Sci Rep. 2021 Nov 11;11(1):22076. doi: 10.1038/s41598-021-01359-3. Sci Rep. 2021. PMID: 34764323 Free PMC article.
-
Adhesive and migratory effects of phosphophoryn are modulated by flanking peptides of the integrin binding motif.PLoS One. 2014 Nov 14;9(11):e112490. doi: 10.1371/journal.pone.0112490. eCollection 2014. PLoS One. 2014. PMID: 25396425 Free PMC article.
-
TRPM8 Channel Promotes the Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells.Front Cell Dev Biol. 2021 Feb 4;9:592946. doi: 10.3389/fcell.2021.592946. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33614639 Free PMC article.
-
Ca2+/calmodulin-dependent protein kinase II regulates the inflammatory hDPSCs dentino-differentiation via BDNF/TrkB receptor signaling.Front Cell Dev Biol. 2025 Mar 26;13:1558736. doi: 10.3389/fcell.2025.1558736. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40206401 Free PMC article.
References
-
- Veis A., Perry A. (1967) The phosphoprotein of the dentin matrix. Biochemistry 6, 2409–2416 - PubMed
-
- Ogbureke K. U., Fisher L. W. (2004) Expression of SIBLINGs and their partner MMPs in salivary glands. J. Dent. Res. 83, 664–670 - PubMed
-
- Linde A., Bhown M., Butler W. T. (1980) Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J. Biol. Chem. 255, 5931–5942 - PubMed
-
- Richardson W. S., Munksgaard E. C., Butler W. T. (1978) Rat incisor phosphoprotein. J. Biol. Chem. 253, 8042–8046 - PubMed
-
- Feng J. Q., Luan X., Wallace J., Jing D., Ohshima T., Kulkarni A. B., D'Souza R. N., Kozak C. A., MacDougall M. (1998) Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J. Biol. Chem. 273, 9457–9464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

