15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat
- PMID: 23362317
- PMCID: PMC3883579
- DOI: 10.2967/jnumed.112.111336
15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat
Abstract
Although it has been believed that brown adipose tissue (BAT) depots disappear shortly after the perinatal period in humans, PET imaging using the glucose analog (18)F-FDG has shown unequivocally the existence of functional BAT in adult humans, suggesting that many humans retain some functional BAT past infancy. The objective of this study was to determine to what extent BAT thermogenesis is activated in adults during cold stress and to establish the relationship between BAT oxidative metabolism and (18)F-FDG tracer uptake.
Methods: Twenty-five healthy adults (15 women and 10 men; mean age ± SD, 30 ± 7 y) underwent triple-oxygen scans (H2(15)O, C(15)O, and (15)O2) as well as measurements of daily energy expenditure (DEE; kcal/d) both at rest and after exposure to mild cold (15.5°C [60°F]) using indirect calorimetry. The subjects were divided into 2 groups (high BAT and low BAT) based on the presence or absence of (18)F-FDG tracer uptake (standardized uptake value [SUV] > 2) in cervical-supraclavicular BAT. Blood flow and oxygen extraction fraction (OEF) were calculated from dynamic PET scans at the location of BAT, muscle, and white adipose tissue. Regional blood oxygen saturation was determined by near-infrared spectroscopy. The total energy expenditure during rest and mild cold stress was measured by indirect calorimetry. Tissue-level metabolic rate of oxygen (MRO2) in BAT was determined and used to calculate the contribution of activated BAT to DEE.
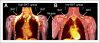
Results: The mass of activated BAT was 59.1 ± 17.5 g (range, 32-85 g) in the high-BAT group (8 women and 1 man; mean age, 29.6 ± 5.5 y) and 2.2 ± 3.6 g (range, 0-9.3 g) in the low-BAT group (9 men and 7 women; mean age, 31.4 ± 10 y). Corresponding maximal SUVs were significantly higher in the high-BAT group than in the low-BAT group (10.7 ± 3.9 vs. 2.1 ± 0.7, P = 0.01). Blood flow values were significantly higher in the high-BAT group than in the low-BAT group for BAT (12.9 ± 4.1 vs. 5.9 ± 2.2 mL/100 g/min, P = 0.03) and white adipose tissue (7.2 ± 3.4 vs. 5.7 ± 2.3 mL/100 g/min, P = 0.03) but were similar for muscle (4.4 ± 1.9 vs. 3.9 ± 1.7 mL/100 g/min). Moreover, OEF in BAT was similar in the 2 groups (0.51 ± 0.17 in high-BAT group vs. 0.47 ± 0.18 in low-BAT group, P = 0.39). During mild cold stress, calculated MRO2 values in BAT increased from 0.97 ± 0.53 to 1.42 ± 0.68 mL/100 g/min (P = 0.04) in the high-BAT group and were significantly higher than those determined in the low-BAT group (0.40 ± 0.28 vs. 0.51 ± 0.23, P = 0.67). The increase in DEE associated with BAT oxidative metabolism was highly variable in the high-BAT group, with an average of 3.2 ± 2.4 kcal/d (range, 1.9-4.6 kcal/d) at rest, and increased to 6.3 ± 3.5 kcal/d (range, 4.0-9.9 kcal/d) during exposure to mild cold. Although BAT accounted for only a small fraction of the cold-induced increase in DEE, such increases were not observed in subjects lacking BAT.
Conclusion: Mild cold-induced thermogenesis in BAT accounts for 15-25 kcal/d in subjects with relatively large BAT depots. Thus, although the presence of active BAT is correlated with cold-induced energy expenditure, direct measurement of MRO2 indicates that BAT is a minor source of thermogenesis in humans.
Figures






References
-
- Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–270. - PubMed
-
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. - PubMed
-
- Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965;206:201–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
