Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines
- PMID: 23362320
- PMCID: PMC3560528
- DOI: 10.1128/mBio.00526-12
Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines
Abstract
Candida albicans has developmental programs that govern transitions between yeast and filamentous morphologies and between unattached and biofilm lifestyles. Here, we report that filamentation, intercellular adherence, and biofilm development were inhibited during interactions between Candida albicans and Pseudomonas aeruginosa through the action of P. aeruginosa-produced phenazines. While phenazines are toxic to C. albicans at millimolar concentrations, we found that lower concentrations of any of three different phenazines (pyocyanin, phenazine methosulfate, and phenazine-1-carboxylate) allowed growth but affected the development of C. albicans wrinkled colony biofilms and inhibited the fungal yeast-to-filament transition. Phenazines impaired C. albicans growth on nonfermentable carbon sources and led to increased production of fermentation products (ethanol, glycerol, and acetate) in glucose-containing medium, leading us to propose that phenazines specifically inhibited respiration. Methylene blue, another inhibitor of respiration, also prevented the formation of structured colony biofilms. The inhibition of filamentation and colony wrinkling was not solely due to lowered extracellular pH induced by fermentation. Compared to smooth, unstructured colonies, wrinkled colony biofilms had higher oxygen concentrations within the colony, and wrinkled regions of these colonies had higher levels of respiration. Together, our data suggest that the structure of the fungal biofilm promotes access to oxygen and enhances respiratory metabolism and that the perturbation of respiration by bacterial molecules such as phenazines or compounds with similar activities disrupts these pathways. These findings may suggest new ways to limit fungal biofilms in the context of disease. IMPORTANCE Many of the infections caused by Candida albicans, a major human opportunistic fungal pathogen, involve both morphological transitions and the formation of surface-associated biofilms. Through the study of C. albicans interactions with the bacterium Pseudomonas aeruginosa, which often coinfects with C. albicans, we have found that P. aeruginosa-produced phenazines modulate C. albicans metabolism and, through these metabolic effects, impact cellular morphology, cell-cell interactions, and biofilm formation. We suggest that the structure of C. albicans biofilms promotes access to oxygen and enhances respiratory metabolism and that the perturbation of respiration by phenazines inhibits biofilm development. Our findings not only provide insight into interactions between these species but also provide valuable insights into novel pathways that could lead to the development of new therapies to treat C. albicans infections.
Figures





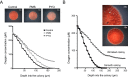
References
-
- Odds FC. 1987. Candida infections: an overview. Crit. Rev. Microbiol. 15:1–5 - PubMed
-
- Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 16:737–748 - PubMed
-
- Nett J, Andes D. 2006. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr. Opin. Microbiol. 9:340–345 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
