Hydroxyl ion addition to one-electron oxidized thymine: unimolecular interconversion of C5 to C6 OH-adducts
- PMID: 23362972
- PMCID: PMC3616772
- DOI: 10.1021/ja310650n
Hydroxyl ion addition to one-electron oxidized thymine: unimolecular interconversion of C5 to C6 OH-adducts
Abstract
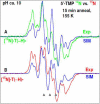
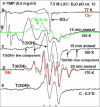
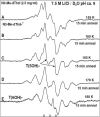
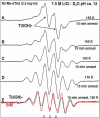
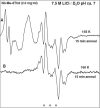
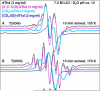
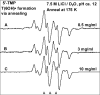
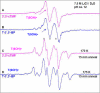
In this work, addition of OH(-) to one-electron oxidized thymidine (dThd) and thymine nucleotides in basic aqueous glasses is investigated. At pHs ca. 9-10 where the thymine base is largely deprotonated at N3, one-electron oxidation of the thymine base by Cl(2)(•-) at ca. 155 K results in formation of a neutral thyminyl radical, T(-H)·. Assignment to T(-H)· is confirmed by employing (15)N substituted 5'-TMP. At pH ≥ ca. 11.5, formation of the 5-hydroxythymin-6-yl radical, T(5OH)·, is identified as a metastable intermediate produced by OH(-) addition to T(-H)· at C5 at ca. 155 K. Upon further annealing to ca. 170 K, T(5OH)· readily converts to the 6-hydroxythymin-5-yl radical, T(6OH)·. One-electron oxidation of N3-methyl-thymidine (N3-Me-dThd) by Cl(2)(•-) at ca. 155 K produces the cation radical (N3-Me-dThd(•+)) for which we find a pH dependent competition between deprotonation from the methyl group at C5 and addition of OH(-) to C5. At pH 7, the 5-methyl deprotonated species is found; however, at pH ca. 9, N3-Me-dThd(•+) produces T(5OH)· that on annealing up to 180 K forms T(6OH)·. Through use of deuterium substitution at C5' and on the thymine base, that is, specifically employing [5',5"-D,D]-5'-dThd, [5',5"-D,D]-5'-TMP, [CD(3)]-dThd and [CD(3),6D]-dThd, we find unequivocal evidence for T(5OH)· formation and its conversion to T(6OH)·. The addition of OH(-) to the C5 position in T(-H)· and N3-Me-dThd(•+) is governed by spin and charge localization. DFT calculations predict that the conversion of the "reducing" T(5OH)· to the "oxidizing" T(6OH)· occurs by a unimolecular OH group transfer from C5 to C6 in the thymine base. The T(5OH)· to T(6OH)· conversion is found to occur more readily for deprotonated dThd and its nucleotides than for N3-Me-dThd. In agreement, calculations predict that the deprotonated thymine base has a lower energy barrier (ca. 6 kcal/mol) for OH transfer than its corresponding N3-protonated thymine base (14 kcal/mol).
Figures


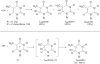
 ) and in PCM (
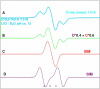
) and in PCM ( ). The variation of spin density distribution along the PES is also shown at some chosen points (see supporting information Table T1).
). The variation of spin density distribution along the PES is also shown at some chosen points (see supporting information Table T1).
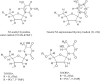
 ) and in PCM (
) and in PCM ( ). The variation of spin density distribution along the PES is also shown at some chosen points, for details see supporting information Table T1.
). The variation of spin density distribution along the PES is also shown at some chosen points, for details see supporting information Table T1.


References
-
- von Sonntag C. Free-radical-induced DNA Damage and Its Repair. Springer-Verlag; Berlin, Heidelberg: 2006. pp. 222–227.
-
- von Sonntag C. Adv. Quantum Chem. 2007;52:5–20.
-
- Myers LS, Jr., Hollis LM, Theard LM, Peterson FC, Warnick A. J. Am. Chem. Soc. 1970;92:2875–2882. - PubMed
-
- Fujita S, Steenken S. J. Am. Chem. Soc. 1981;103:2540–2545.
-
- Al-Sheikhly M, von Sonntag C. Z. Naturforsch. 1983;38b:1622–1629.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

