Quantifying effect of intraplaque hemorrhage on critical plaque wall stress in human atherosclerotic plaques using three-dimensional fluid-structure interaction models
- PMID: 23363206
- PMCID: PMC5418542
- DOI: 10.1115/1.4007954
Quantifying effect of intraplaque hemorrhage on critical plaque wall stress in human atherosclerotic plaques using three-dimensional fluid-structure interaction models
Abstract
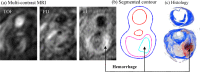
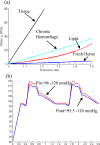
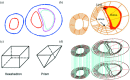
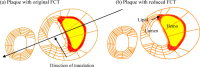
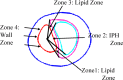
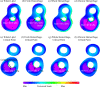
Recent magnetic resonance studies have indicated that intraplaque hemorrhage (IPH) may accelerate plaque progression and play an important role in plaque destabilization. However, the impact of hemorrhage on critical plaque wall stress (CPWS) and strain (CPWSn) has yet to be determined. The objective of this study was to assess the effect of the presence and size of IPH on wall mechanics. The magnetic resonance image (MRI) of one patient with histology-confirmed IPH was used to build eight 3D fluid-structure interaction (FSI) models by altering the dimensions of the existing IPH. As a secondary end point, the combined effect of IPH and fibrous cap thickness (FCT) was assessed. A volume curve fitting method (VCFM) was applied to generate a mesh that would guarantee numerical convergence. Plaque wall stress (PWS), strain (PWSn), and flow shear stress (FSS) were extracted from all nodal points on the lumen surface for analysis. Keeping other conditions unchanged, the presence of intraplaque hemorrhage caused a significant increase (27.5%) in CPWS; reduced FCT caused an increase of 22.6% of CPWS. Similar results were found for CPWSn. Furthermore, combination of IPH presence, reduced FCT, and increased IPH volume caused an 85% and 75% increase in CPWS and CPWSn, respectively. These results show that intraplaque hemorrhage has considerable impact on plaque stress and strain conditions and accurate quantification of IPH could lead to more accurate assessment of plaque vulnerability. Large-scale studies are needed to further validate our findings.
Figures



References
-
- Naghavi, M. , Libby, P. , Falk, E. , Casscells, S. W. , Litovsky, S. , Rumberger, J. , Badimon, J. J. , Stefanadis, C. , Moreno, P. , Pasterkamp, G. , Fayad, Z. , Stone, P. H. , Waxman, S. , Raggi, P. , Madjid, M. , Zarrabi, A. , Burke, A. , Yuan, C. , Fitzgerald, P. J. , Siscovick, D. S. , de Korte, C. L. , Aikawa, M. , Juhani Airaksinen, K. E. , Assmann, G. , Becker, C. R. , Chesebro, J. H. , Farb, A. , Galis, Z. S. , Jackson, C. , Jang, I. K. , Koenig, W. , Lodder, R. A. , March, K. , Demirovic, J. , Navab, M. , Priori, S. G. , Rekhter, M. D. , Bahr, R. , Grundy, S. M. , Mehran, R. , Colombo, A. , Boerwinkle, E. , Ballantyne, C. , Insull, W., Jr. , Schwartz, R. S. , Vogel, R. , Serruys, P. W. , Hansson, G. K. , Faxon, D. P. , Kaul, S. , Drexler, H. , Greenland, P. , Muller, J. E. , Virmani, R. , Ridker, P. M. , Zipes, D. P. , Shah, P. K. , and Willerson, J. T. , 2003, “From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part I,” Circulation, 108(14), pp. 1664–1672.10.1161/01.CIR.0000087480.94275.97 - DOI - PubMed
-
- Naghavi, M. , Libby, P. , Falk, E. , Casscells, S. W. , Litovsky, S. , Rumberger, J. , Badimon, J. J. , Stefanadis, C. , Moreno, P. , Pasterkamp, G. , Fayad, Z. , Stone, P. H. , Waxman, S. , Raggi, P. , Madjid, M. , Zarrabi, A. , Burke, A. , Yuan, C. , Fitzgerald, P. J. , Siscovick, D. S. , de Korte, C. L. , Aikawa, M. , Juhani Airaksinen, K. E. , Assmann, G. , Becker, C. R. , Chesebro, J. H. , Farb, A. , Galis, Z. S. , Jackson, C. , Jang, I. K. , Koenig, W. , Lodder, R. A. , March, K. , Demirovic, J. , Navab, M. , Priori, S. G. , Rekhter, M. D. , Bahr, R. , Grundy, S. M. , Mehran, R. , Colombo, A. , Boerwinkle, E. , Ballantyne, C. , Insull, W., Jr. , Schwartz, R. S. , Vogel, R. , Serruys, P. W. , Hansson, G. K. , Faxon, D. P. , Kaul, S. , Drexler, H. , Greenland, P. , Muller, J. E. , Virmani, R. , Ridker, P. M. , Zipes, D. P. , Shah, P. K. , and Willerson, J. T. , 2003, “From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part II,” Circulation, 108(15), pp. 1772–1778.10.1161/01.CIR.0000087481.55887.C9 - DOI - PubMed
-
- Saam, T. , Ferguson, M. S. , Yarnykh, V. L. , Takaya, N. , Xu, D. , Polissar, N. L. , Hatsukami, T. S. , and Yuan, C. , 2005, “Quantitative Evaluation of Carotid Plaque Composition by In Vivo MRI,” Arterioscler., Thromb., Vasc. Biol., 25(1), pp. 234–239. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

