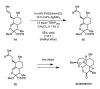
Wacker-type oxidation of internal alkenes using Pd(Quinox) and TBHP
- PMID: 23363387
- PMCID: PMC3615722
- DOI: 10.1021/jo302638v
Wacker-type oxidation of internal alkenes using Pd(Quinox) and TBHP
Abstract
The Pd-catalyzed TBHP-mediated Wacker-type oxidation of internal alkenes is reported. The reaction uses 2-(4,5-dihydro-2-oxazolyl)quinoline (Quinox) as ligand and TBHP(aq) as oxidant to deliver single ketone constitutional isomer products in a predictable fashion from electronically biased olefins. This methodology is showcased through its application on an advanced intermediate in the total synthesis of the antimalarial drug artemisinin.
Figures
References
-
- Morandi B, Wickens ZK, Grubbs RH. Angew. Chem., Int. Ed. 2013 DOI: 10.1002/anie.201209541. - PubMed
- Gaunt MJ, Yu J, Spencer JB. Chem. Commun. 2001;1844 - PubMed
- Nelson DJ, Li R, Brammer C. J. Am. Chem. Soc. 2001;123:1564. - PubMed
- Betzemeier B, Lhermitte F. d. r., Knochel P. Tetrahedron Lett. 1998;39:6667.
- Okota T, Fujibayashi S, Nishiyama Y, Sakaguchi S, Ishii Y. J. Mol. Catal. A: Chem. 1996;114:113.
- Kang S-K, Jung K-Y, Chung J-U, Namkoong E-Y, Kim T-H. J. Org. Chem. 1995;60:4678.
- Sommovigo M, Alper H. J. Mol. Catal. 1994;88:151.
- Hosokawa T, Nakahira T, Takano M, Murahashi S-I. J. Mol. Catal. 1992;74:489.
- Miller DG, Wayner DDM. Can. J. Chem. 1992;70:2485.
- Kulkarni MG, Sebastian MT. Synth. Commun. 1991;21:581.
- Miller DG, Wayner DDM. J. Org. Chem. 1990;55:2924.
- Takehira K, Hayakawa T, Orita H, Shimizu M. J. Mol. Catal. 1989;53:15.
- Grattan TJ, Whitehurst JS. J. Chem. Soc., Chem. Commun. 1988;43
- Akermark B, Soederberg BC, Hall SS. Organometallics. 1987;6:2608.
- Takehira K, Orita H, Oh IH, Leobardo CO, Martinez GC, Shimidzu M, Hayakawa T, Ishikawa T. J. Mol. Catal. 1987;42:247.
- Tsuji J, Minato M. Tetrahedron Lett. 1987;28:3683.
- Keinan E, Seth KK, Lamed R. J. Am. Chem. Soc. 1986;108:3474.
- Zahalka HA, Januszkiewicz K, Alper H. J. Mol. Catal. 1986;35:249.
- Alper H, Januszkiewicz K, Smith DJH. Tetrahedron Lett. 1985;26:2263.
- Takehira K, Hayakawa T, Orita H. Chem. Lett. 1985;14:1835.
- Ogawa H, Fujinami H, Taya K, Teratani S. Bull. Chem. Soc. Jpn. 1984;57:1908.
-
- Tsuji J, Nagashima H, Hori K. Tetrahedron Lett. 1982;23:2679.
- Tsuji J, Nagashima H, Hori K. Chem. Lett. 1980;9:257.
-
- Weiner B, Baeza A, Jerphagnon T, Feringa BL. J. Am. Chem. Soc. 2009;131:9473. - PubMed
-
- Mitsudome T, Mizumoto K, Mizugaki T, Jitsukawa K, Kaneda K. Angew. Chem. Int. Ed. 2010;49:1238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources