RNAi-mediated knockdown of Xist does not rescue the impaired development of female cloned mouse embryos
- PMID: 23363561
- PMCID: PMC3934135
- DOI: 10.1262/jrd.2012-195
RNAi-mediated knockdown of Xist does not rescue the impaired development of female cloned mouse embryos
Abstract
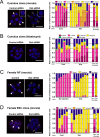
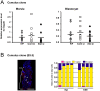
In mice, one of the major epigenetic errors associated with somatic cell nuclear transfer (SCNT) is ectopic expression of Xist during the preimplantation period in both sexes. We found that this aberrant Xist expression could be impeded by deletion of Xist from the putative active X chromosome in donor cells. In male clones, it was also found that prior injection of Xist-specific siRNA could significantly improve the postimplantation development of cloned embryos as a result of a significant repression of Xist at the morula stage. In this study, we examined whether the same knockdown strategy could work as well in female SCNT-derived embryos. Embryos were reconstructed with cumulus cell nuclei and injected with Xist-specific siRNA at 6-7 h after oocyte activation. RNA FISH analysis revealed that siRNA treatment successfully repressed Xist RNA at the morula stage, as shown by the significant decrease in the number of cloud-type Xist signals in the blastomere nuclei. However, blastomeres with different sizes (from "pinpoint" to "cloud") and numbers of Xist RNA signals remained within single embryos. After implantation, the dysregulated Xist expression was normalized autonomously, as in male clones, to a state of monoallelic expression in both embryonic and extraembryonic tissues. However, at term there was no significant improvement in the survival of the siRNA-injected cloned embryos. Thus, siRNA injection was largely effective in repressing the Xist overexpression in female cloned embryos but failed to rescue them, probably because of an inability to mimic consistent monoallelic Xist expression in these embryos. This could only be achieved in female embryos by applying a gene knockout strategy rather than an siRNA approach.
Figures
Similar articles
-
Improvement of developmental competence of cloned male pig embryos by short hairpin ribonucleic acid (shRNA) vector-based but not small interfering RNA (siRNA)-mediated RNA interference (RNAi) of Xist expression.J Reprod Dev. 2019 Dec 18;65(6):533-539. doi: 10.1262/jrd.2019-070. Epub 2019 Oct 21. J Reprod Dev. 2019. PMID: 31631092 Free PMC article.
-
RNAi-mediated knockdown of Xist can rescue the impaired postimplantation development of cloned mouse embryos.Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20621-6. doi: 10.1073/pnas.1112664108. Epub 2011 Nov 7. Proc Natl Acad Sci U S A. 2011. PMID: 22065773 Free PMC article.
-
Effects of RNAi-mediated knockdown of Xist on the developmental efficiency of cloned male porcine embryos.J Reprod Dev. 2016 Dec 20;62(6):591-597. doi: 10.1262/jrd.2016-095. Epub 2016 Aug 29. J Reprod Dev. 2016. PMID: 27569767 Free PMC article.
-
Intrinsic and extrinsic molecular determinants or modulators for epigenetic remodeling and reprogramming of somatic cell-derived genome in mammalian nuclear-transferred oocytes and resultant embryos.Pol J Vet Sci. 2018 Mar;21(1):217-227. doi: 10.24425/119040. Pol J Vet Sci. 2018. PMID: 29624006 Review.
-
Establishment and maintenance of random monoallelic expression.Development. 2024 May 15;151(10):dev201741. doi: 10.1242/dev.201741. Epub 2024 May 30. Development. 2024. PMID: 38813842 Free PMC article. Review.
Cited by
-
RNAi-mediated knockdown of Parp1 does not improve the development of female cloned mouse embryos.Oncotarget. 2017 Jul 18;8(41):69863-69873. doi: 10.18632/oncotarget.19418. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050247 Free PMC article.
-
Epigenetic modification with trichostatin A does not correct specific errors of somatic cell nuclear transfer at the transcriptomic level; highlighting the non-random nature of oocyte-mediated reprogramming errors.BMC Genomics. 2016 Jan 4;17:16. doi: 10.1186/s12864-015-2264-z. BMC Genomics. 2016. PMID: 26725231 Free PMC article.
-
Improvement of developmental competence of cloned male pig embryos by short hairpin ribonucleic acid (shRNA) vector-based but not small interfering RNA (siRNA)-mediated RNA interference (RNAi) of Xist expression.J Reprod Dev. 2019 Dec 18;65(6):533-539. doi: 10.1262/jrd.2019-070. Epub 2019 Oct 21. J Reprod Dev. 2019. PMID: 31631092 Free PMC article.
-
Development of reproductive engineering techniques at the RIKEN BioResource Center.Exp Anim. 2017 Jan 27;66(1):1-16. doi: 10.1538/expanim.16-0074. Epub 2016 Oct 19. Exp Anim. 2017. PMID: 27760894 Free PMC article. Review.
-
XIST Derepression in Active X Chromosome Hinders Pig Somatic Cell Nuclear Transfer.Stem Cell Reports. 2018 Feb 13;10(2):494-508. doi: 10.1016/j.stemcr.2017.12.015. Epub 2018 Jan 11. Stem Cell Reports. 2018. PMID: 29337117 Free PMC article.
References
-
- Ogura A, Inoue K, Ogonuki N, Lee J, Kohda T, Ishino F. Phenotypic effects of somatic cell cloning in the mouse. Cloning Stem Cells 2002; 4: 397–405 - PubMed
-
- Meissner A, Jaenisch R. Mammalian nuclear transfer. Dev Dyn 2006; 235: 2460–2469 - PubMed
-
- Thuan NV, Kishigami S, Wakayama T. How to improve the success rate of mouse cloning technology. J Reprod Dev 2010; 56: 20–30 - PubMed
-
- Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun 2006; 340: 183–189 - PubMed
-
- Rybouchkin A, Kato Y, Tsunoda Y. Role of histone acetylation in reprogramming of somatic nuclei following nuclear transfer. Biol Reprod 2006; 74: 1083–1089 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

