Characterisation of fibroblast-like synoviocytes from a murine model of joint inflammation
- PMID: 23363614
- PMCID: PMC3672796
- DOI: 10.1186/ar4158
Characterisation of fibroblast-like synoviocytes from a murine model of joint inflammation
Abstract
Introduction: Fibroblast-like synoviocytes (FLS) play a central role in defining the stromal environment in inflammatory joint diseases. Despite a growing use of FLS isolated from murine inflammatory models, a detailed characterisation of these cells has not been performed.
Methods: In this study, FLS were isolated from inflamed joints of mice expressing both the T cell receptor transgene KRN and the MHC class II molecule Ag7 (K/BxN mice) and their purity in culture determined by immunofluorescence and real-time reverse transcription polymerase chain reaction (real-time RT-PCR). Basal expression of proinflammatory genes was determined by real-time RT-PCR. Secreted interleukin 6 (IL-6) was measured by enzyme-linked immunosorbent assay (ELISA), and its regulation by tumor necrosis factor-alpha (TNF-α and corticosterone (the major glucocorticoid in rodents) measured relative to other mesenchymal cell populations.
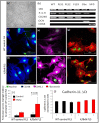
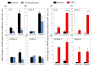
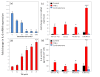
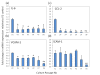
Results: Purity of FLS culture was identified by positive expression of fibronectin, prolyl 4-hydroxylase, cluster of differentiation 90.2 (CD90.2) and 248 (CD248) in greater than 98% of the population. Cultured FLS were able to migrate and invade through matrigel, a process enhanced in the presence of TNF-α. FLS isolated from K/BxN mice possessed significantly greater basal expression of the inflammatory markers IL-6, chemokine ligand 2 (CCL-2) and vascular cell adhesion molecule 1 (VCAM-1) when compared to FLS isolated from non-inflamed tissue (IL-6, 3.6 fold; CCL-2, 11.2 fold; VCAM-1, 9 fold; P<0.05). This elevated expression was abrogated in the presence of corticosterone at 100 nmol/l. TNF-α significantly increased expression of all inflammatory markers to a much greater degree in K/BxN FLS relative to other mesenchymal cell lines (K/BxN; IL-6, 40.8 fold; CCL-2, 1343.2 fold; VCAM-1, 17.8 fold; ICAM-1, 13.8 fold; P<0.05), with secreted IL-6 mirroring these results (K/BxN; con, 169±29.7 versus TNF-α, 923±378.8 pg/ml/1×10⁵ cells; P<0.05). Dose response experiments confirmed effective concentrations between 10 and 100 nmol/l for corticosterone and 1 and 10 ng/ml for TNF-α, whilst inflammatory gene expression in FLS was shown to be stable between passages four and seven.
Conclusions: This study has established a well characterised set of key inflammatory genes for in vitro FLS culture, isolated from K/BxN mice and non-inflamed wild-type controls. Their response to both pro- and anti-inflammatory signalling has been assessed and shown to strongly resemble that which is seen in human FLS culture. Additionally, this study provides guidelines for the effective characterisation, duration and treatment of murine FLS culture.
Figures

References
-
- Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956;91:95–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

