Transcription and redox enzyme activities: comparison of equilibrium and disequilibrium levels in the three-spined stickleback
- PMID: 23363636
- PMCID: PMC3574399
- DOI: 10.1098/rspb.2012.2974
Transcription and redox enzyme activities: comparison of equilibrium and disequilibrium levels in the three-spined stickleback
Abstract
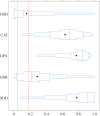
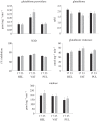
Evolutionary and acclimatory responses require functional variability, but in contrast with mRNA and protein abundance data, most physiological measurements cannot be obtained in a high-throughput manner. Consequently, one must either rely on high-throughput transcriptomic or proteomic data with only predicted functional information, or accept the limitation that most physiological measurements can give fewer data than those provided by transcriptomics or proteomics. We evaluated how transcriptional and redox enzyme activity data agreed with regard to population differentiation (i.e. a system in steady state in which any time lag between transcription, translation and post-translational effects would be irrelevant) and in response to an acute 6°C increase in temperature (i.e. a disequilibrium state wherein translation could not have caught up with transcription) in the three-spined stickleback (Gasterosteus aculeatus). Transcriptional and enzyme activity data corresponded well with regard to population differentiation, but less so with regard to acute temperature increase. The data thus suggest that transcriptional and functional measurements can lead to similar conclusions when a biological system is in a steady state. The responses to acute changes must, as has been demonstrated earlier, be based on changes in cellular conditions or properties of existing proteins without significant de novo synthesis of new gene products.
Figures


References
-
- Furlong EEM. 2011. Molecular biology: a fly in the face of genomics. Nature 471, 458–45910.1038/471458a (doi:10.1038/471458a) - DOI - DOI - PubMed
-
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. 2011. Global quantification of mammalian gene expression control. Nature 473, 337–34210.1038/nature10098 (doi:10.1038/nature10098) - DOI - DOI - PubMed
-
- Nikinmaa M, Waser W. 2007. Molecular and cellular studies in evolutionary physiology of natural vertebrate populations: influences of individual variation and genetic components on sampling and measurements. J. Exp. Biol. 210, 1847–185710.1242/jeb.002717 (doi:10.1242/jeb.002717) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
