Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial
- PMID: 23363666
- PMCID: PMC4128170
- DOI: 10.1016/S0140-6736(12)61687-0
Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial
Abstract
Background: Bacteraemia is an important cause of morbidity and mortality in critically ill children. Our objective was to assess whether daily bathing in chlorhexidine gluconate (CHG) compared with standard bathing practices would reduce bacteraemia in critically ill children.
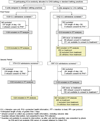
Methods: In an unmasked, cluster-randomised, two-period crossover trial, ten paediatric intensive-care units at five hospitals in the USA were randomly assigned a daily bathing routine for admitted patients older than 2 months, either standard bathing practices or using a cloth impregnated with 2% CHG, for a 6-month period. Units switched to the alternative bathing method for a second 6-month period. 6482 admissions were screened for eligibility. The primary outcome was an episode of bacteraemia. We did intention-to-treat (ITT) and per-protocol (PP) analyses. This study is registered with ClinicalTrials.gov (identifier NCT00549393).
Findings: 1521 admitted patients were excluded because their length of stay was less than 2 days, and 14 refused to participate. 4947 admissions were eligible for analysis. In the ITT population, a non-significant reduction in incidence of bacteraemia was noted with CHG bathing (3·52 per 1000 days, 95% CI 2·64-4·61) compared with standard practices (4·93 per 1000 days, 3·91-6·15; adjusted incidence rate ratio [aIRR] 0·71, 95% CI 0·42-1·20). In the PP population, incidence of bacteraemia was lower in patients receiving CHG bathing (3·28 per 1000 days, 2·27-4·58) compared with standard practices (4·93 per 1000 days, 3·91-6·15; aIRR 0·64, 0·42-0·98). No serious study-related adverse events were recorded, and the incidence of CHG-associated skin reactions was 1·2 per 1000 days (95% CI 0·60-2·02).
Interpretation: Critically ill children receiving daily CHG bathing had a lower incidence of bacteraemia compared with those receiving a standard bathing routine. Furthermore, the treatment was well tolerated.
Funding: Sage Products, US National Institutes of Health.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
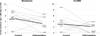
Comment in
-
Rethinking infection prevention research.Lancet. 2013 Mar 30;381(9872):1078-9. doi: 10.1016/S0140-6736(12)61996-5. Epub 2013 Jan 28. Lancet. 2013. PMID: 23363665 No abstract available.
References
-
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271(20):1598–1601. - PubMed
-
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37(10):783–805. - PubMed
-
- Elward AM, Hollenbeak CS, Warren DK, Fraser VJ. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2005;115(4):868–872. - PubMed
-
- Armenian SH, Singh J, Arrieta AC. Risk factors for mortality resulting from bloodstream infections in a pediatric intensive care unit. Pediatr Infect Dis J. 2005;24(4):309–314. - PubMed
-
- Gray J, Gossain S, Morris K. Three-year survey of bacteremia and fungemia in a pediatric intensive care unit. Pediatr Infect Dis J. 2001;20(4):416–421. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

