Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution
- PMID: 23363705
- PMCID: PMC4053949
- DOI: 10.1186/gb-2013-14-1-r10
Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution
Abstract
Background: Centromeres are essential for chromosome segregation, yet their DNA sequences evolve rapidly. In most animals and plants that have been studied, centromeres contain megabase-scale arrays of tandem repeats. Despite their importance, very little is known about the degree to which centromere tandem repeats share common properties between different species across different phyla. We used bioinformatic methods to identify high-copy tandem repeats from 282 species using publicly available genomic sequence and our own data.
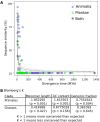
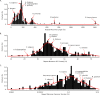
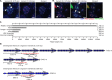
Results: Our methods are compatible with all current sequencing technologies. Long Pacific Biosciences sequence reads allowed us to find tandem repeat monomers up to 1,419 bp. We assumed that the most abundant tandem repeat is the centromere DNA, which was true for most species whose centromeres have been previously characterized, suggesting this is a general property of genomes. High-copy centromere tandem repeats were found in almost all animal and plant genomes, but repeat monomers were highly variable in sequence composition and length. Furthermore, phylogenetic analysis of sequence homology showed little evidence of sequence conservation beyond approximately 50 million years of divergence. We find that despite an overall lack of sequence conservation, centromere tandem repeats from diverse species showed similar modes of evolution.
Conclusions: While centromere position in most eukaryotes is epigenetically determined, our results indicate that tandem repeats are highly prevalent at centromeres of both animal and plant genomes. This suggests a functional role for such repeats, perhaps in promoting concerted evolution of centromere DNA across chromosomes.
Figures