Cytoplasmic free Ca2+ is essential for multiple steps in malaria parasite egress from infected erythrocytes
- PMID: 23363708
- PMCID: PMC3564835
- DOI: 10.1186/1475-2875-12-41
Cytoplasmic free Ca2+ is essential for multiple steps in malaria parasite egress from infected erythrocytes
Abstract
Background: Egress of Plasmodium falciparum, from erythrocytes at the end of its asexual cycle and subsequent parasite invasion into new host cells, is responsible for parasite dissemination in the human body. The egress pathway is emerging as a coordinated multistep programme that extends in time for tens of minutes, ending with rapid parasite extrusion from erythrocytes. While the Ca2+ regulation of the invasion of P. falciparum in erythrocytes is well established, the role of Ca2+ in parasite egress is poorly understood. This study analysed the involvement of cytoplasmic free Ca2+ in infected erythrocytes during the multistep egress programme of malaria parasites.
Methods: Live-cell fluorescence microscopy was used to image parasite egress from infected erythrocytes, assessing the effect of drugs modulating Ca2+ homeostasis on the egress programme.
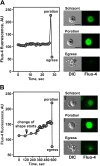
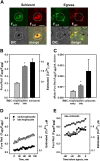
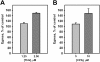
Results: A steady increase in cytoplasmic free Ca2+ is found to precede parasite egress. This increase is independent of extracellular Ca2+ for at least the last two hours of the cycle, but is dependent upon Ca2+ release from internal stores. Intracellular BAPTA chelation of Ca2+ within the last 45 minutes of the cycle inhibits egress prior to parasitophorous vacuole swelling and erythrocyte membrane poration, two characteristic morphological transformations preceding parasite egress. Inhibitors of the parasite endoplasmic reticulum (ER) Ca2+-ATPase accelerate parasite egress, indicating that Ca2+ stores within the ER are sufficient in supporting egress. Markedly accelerated egress of apparently viable parasites was achieved in mature schizonts using Ca2+ ionophore A23187. Ionophore treatment overcomes the BAPTA-induced block of parasite egress, confirming that free Ca2+ is essential in egress initiation. Ionophore treatment of immature schizonts had an adverse effect inducing parasitophorous vacuole swelling and killing the parasites within the host cell.
Conclusions: The parasite egress programme requires intracellular free Ca2+ for egress initiation, vacuole swelling, and host cell cytoskeleton digestion. The evidence that parasitophorous vacuole swelling, a stage of unaffected egress, is dependent upon a rise in intracellular Ca2+ suggests a mechanism for ionophore-inducible egress and a new target for Ca2+ in the programme liberating parasites from the host cell. A regulatory pathway for egress that depends upon increases in intracellular free Ca2+ is proposed.
Figures
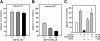

Similar articles
-
New stages in the program of malaria parasite egress imaged in normal and sickle erythrocytes.Curr Biol. 2010 Jun 22;20(12):1117-21. doi: 10.1016/j.cub.2010.04.051. Epub 2010 May 27. Curr Biol. 2010. PMID: 20537541 Free PMC article.
-
The malaria parasite PP1 phosphatase controls the initiation of the egress pathway of asexual blood-stages by regulating the rounding-up of the vacuole.PLoS Pathog. 2025 Jan 14;21(1):e1012455. doi: 10.1371/journal.ppat.1012455. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39808636 Free PMC article.
-
Ca(2+) -mediated exocytosis of subtilisin-like protease 1: a key step in egress of Plasmodium falciparum merozoites.Cell Microbiol. 2013 Jun;15(6):910-21. doi: 10.1111/cmi.12086. Epub 2012 Dec 28. Cell Microbiol. 2013. PMID: 23217145
-
Ion metabolism in malaria-infected erythrocytes.Blood Cells. 1990;16(2-3):437-49. Blood Cells. 1990. PMID: 2175223 Review.
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
Cited by
-
A reference document on Permissible Limits for solvents and buffers during in vitro antimalarial screening.Sci Rep. 2018 Oct 8;8(1):14974. doi: 10.1038/s41598-018-33226-z. Sci Rep. 2018. PMID: 30297791 Free PMC article.
-
A kinetic fluorescence assay reveals unusual features of Ca⁺⁺ uptake in Plasmodium falciparum-infected erythrocytes.Malar J. 2014 May 18;13:184. doi: 10.1186/1475-2875-13-184. Malar J. 2014. PMID: 24885754 Free PMC article.
-
Recent insights into apicomplexan parasite egress provide new views to a kill.Curr Opin Microbiol. 2013 Aug;16(4):459-64. doi: 10.1016/j.mib.2013.04.008. Epub 2013 May 28. Curr Opin Microbiol. 2013. PMID: 23725669 Free PMC article. Review.
-
Multiple genetic loci define Ca++ utilization by bloodstream malaria parasites.BMC Genomics. 2019 Jan 16;20(1):47. doi: 10.1186/s12864-018-5418-y. BMC Genomics. 2019. PMID: 30651090 Free PMC article.
-
Signaling Strategies of Malaria Parasite for Its Survival, Proliferation, and Infection during Erythrocytic Stage.Front Immunol. 2017 Mar 28;8:349. doi: 10.3389/fimmu.2017.00349. eCollection 2017. Front Immunol. 2017. PMID: 28400771 Free PMC article. Review.
References
-
- Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, Baker DA, Wandless TJ, Duraisingh MT. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

