Exocytosis of neutrophil granule subsets and activation of prolyl isomerase 1 are required for respiratory burst priming
- PMID: 23363774
- PMCID: PMC3691856
- DOI: 10.1159/000345992
Exocytosis of neutrophil granule subsets and activation of prolyl isomerase 1 are required for respiratory burst priming
Abstract
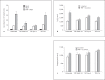
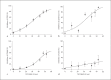
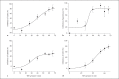
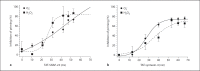
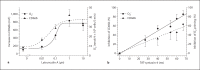
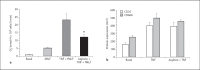
This study tested the hypothesis that priming the neutrophil respiratory burst requires both granule exocytosis and activation of the prolyl isomerase Pin1. Fusion proteins containing the TAT cell permeability sequence and either the SNARE domain of syntaxin-4 or the N-terminal SNARE domain of SNAP-23 were used to examine the role of granule subsets in TNF-mediated respiratory burst priming using human neutrophils. Concentration-inhibition curves for exocytosis of individual granule subsets and for priming of fMLF-stimulated superoxide release and phagocytosis-stimulated H2O2 production were generated. Maximal inhibition of priming ranged from 72 to 88%. Linear regression lines for inhibition of priming versus inhibition of exocytosis did not differ from the line of identity for secretory vesicles and gelatinase granules, while the slopes or the y-intercepts were different from the line of identity for specific and azurophilic granules. Inhibition of Pin1 reduced priming by 56%, while exocytosis of secretory vesicles and specific granules was not affected. These findings indicate that exocytosis of secretory vesicles and gelatinase granules and activation of Pin1 are independent events required for TNF-mediated priming of neutrophil respiratory burst.
Copyright © 2013 S. Karger AG, Basel.
Figures


References
-
- Condliffe AM, Kitchen E, Clivers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci. 1998;94:461–471. - PubMed
-
- El-Benna J, Dang PM-C, Gougerot-Pocidalo M-A. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. - PubMed
-
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
-
- Sheppard FR, Kelher MR, Moore EE, McLaughlin NJD, Banerjee A, Silliman CG. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

