SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells
- PMID: 23363816
- PMCID: PMC3578140
- DOI: 10.1158/1078-0432.CCR-12-1517
SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells
Abstract
Purpose: Human leukocyte antigen (HLA) class I antigen processing machinery (APM) component downregulation permits escape of malignant cells from recognition by cytotoxic T lymphocytes (CTL) and correlates with poor prognosis in patients with head and neck cancer (HNC). Activated STAT1 (pSTAT1) is necessary for APM component expression in HNC cells. We investigated whether an overexpressed phosphatase was responsible for basal suppression of pSTAT1 and subsequent APM component-mediated immune escape in HNC cells.
Experimental design: Immunohistochemical staining and reverse transcription PCR of paired HNC tumors was performed for the phosphatases src homology domain-containing phosphatase (SHP)-1 and SHP2. Depletion of phosphatase activity in HNC and STAT1(-/-) tumor cells was achieved by siRNA knockdown. HLA class I-restricted, tumor antigen-specific CTL were used in IFN-γ ELISPOT assays against HNC cells. Chemokine secretion was measured after SHP2 depletion in HNC cells.
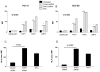
Results: SHP2, but not SHP1, was significantly upregulated in HNC tissues. In HNC cells, SHP2 depletion significantly upregulated expression of pSTAT1 and HLA class I APM components. Overexpression of SHP2 in nonmalignant keratinocytes inhibited IFN-γ-mediated STAT1 phosphorylation, and SHP2 depletion in STAT1(-/-) tumor cells did not significantly induce IFN-γ-mediated APM component expression, verifying STAT1 dependence of SHP2 activity. SHP2 depletion induced recognition of HNC cells by HLA class I-restricted CTL and secretion of inflammatory, T-cell attracting chemokines, RANTES and IP10.
Conclusion: These findings suggest for the first time an important role for SHP2 in APM-mediated escape of HNC cells from CTL recognition. Targeting SHP2 could enhance T-cell-based cancer immunotherapy.
©2012 AACR.
Figures






References
-
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. - PubMed
-
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The Frequency and Suppressor Function of CD4+CD25highFoxp3+ T Cells in the Circulation of Patients with Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2007;13:6301–6311. - PubMed
-
- Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:3755–3762. - PubMed
-
- Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–3409. 2006. - PubMed
-
- Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol Res. 2005;33:113–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

