CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis
- PMID: 23363977
- PMCID: PMC3616630
- DOI: 10.1126/scitranslmed.3004912
CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis
Abstract
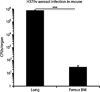
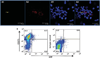
Mycobacterium tuberculosis (Mtb) can persist in hostile intracellular microenvironments evading immune cells and drug treatment. However, the protective cellular niches where Mtb persists remain unclear. We report that Mtb may maintain long-term intracellular viability in a human bone marrow (BM)-derived CD271(+)/CD45(-) mesenchymal stem cell (BM-MSC) population in vitro. We also report that Mtb resides in an equivalent population of BM-MSCs in a mouse model of dormant tuberculosis infection. Viable Mtb was detected in CD271(+)/CD45(-) BM-MSCs isolated from individuals who had successfully completed months of anti-Mtb drug treatment. These results suggest that CD271(+) BM-MSCs may provide a long-term protective intracellular niche in the host in which dormant Mtb can reside.
Figures





References
-
- Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84:29–44. - PubMed
-
- Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17:183–188. - PubMed
-
- Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., III Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008;76:2333–2340. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

