Aftins increase amyloid-β42, lower amyloid-β38, and do not alter amyloid-β40 extracellular production in vitro: toward a chemical model of Alzheimer's disease?
- PMID: 23364140
- PMCID: PMC5039020
- DOI: 10.3233/JAD-121777
Aftins increase amyloid-β42, lower amyloid-β38, and do not alter amyloid-β40 extracellular production in vitro: toward a chemical model of Alzheimer's disease?
Abstract
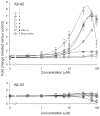
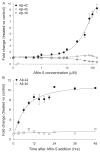
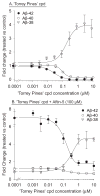
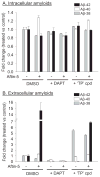
Increased production of amyloid-β (Aβ)42 peptide, derived from the amyloid-β protein precursor, and its subsequent aggregation into oligomers and plaques constitutes a hallmark of Alzheimer's disease (AD). We here report on a family of low molecular weight molecules, the Aftins (Amyloid-β Forty-Two Inducers), which, in cultured cells, dramatically affect the production of extracellular/secreted amyloid peptides. Aftins trigger β-secretase inhibitor and γ-secretase inhibitors (GSIs) sensitive, robust upregulation of Aβ42, and parallel down-regulation of Aβ38, while Aβ40 levels remain stable. In contrast, intracellular levels of these amyloids appear to remain stable. In terms of their effects on Aβ38/Aβ40/Aβ42 relative abundance, Aftins act opposite to γ-secretase modulators (GSMs). Aβ42 upregulation induced by Aftin-5 is unlikely to originate from reduced proteolytic degradation or diminished autophagy. Aftin-5 has little effects on mitochondrial functional parameters (swelling, transmembrane potential loss, cytochrome c release, oxygen consumption) but reversibly alters the ultrastructure of mitochondria. Aftins thus alter the Aβ levels in a fashion similar to that described in the brain of AD patients. Aftins therefore constitute new pharmacological tools to investigate this essential aspect of AD, in cell cultures, allowing (1) the detection of inhibitors of Aftin induced action (potential 'anti-AD compounds', including GSIs and GSMs) but also (2) the identification, in the human chemical exposome, of compounds that, like Aftins, might trigger sustained Aβ42 production and Aβ38 down-regulation (potential 'pro-AD compounds').
Figures
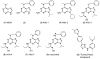


References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. Erratum in: Science 297, 2209. - PubMed
-
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci. 2012;15:349–357. - PubMed
-
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

