Arenavirus reverse genetics for vaccine development
- PMID: 23364194
- PMCID: PMC3709625
- DOI: 10.1099/vir.0.051102-0
Arenavirus reverse genetics for vaccine development
Abstract
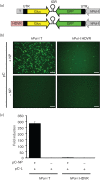
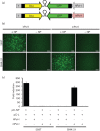
Arenaviruses are important human pathogens with no Food and Drug Administration (FDA)-licensed vaccines available and current antiviral therapy being limited to an off-label use of the nucleoside analogue ribavirin of limited prophylactic efficacy. The development of reverse genetics systems represented a major breakthrough in arenavirus research. However, rescue of recombinant arenaviruses using current reverse genetics systems has been restricted to rodent cells. In this study, we describe the rescue of recombinant arenaviruses from human 293T cells and Vero cells, an FDA-approved line for vaccine development. We also describe the generation of novel vectors that mediate synthesis of both negative-sense genome RNA and positive-sense mRNA species of lymphocytic choriomeningitis virus (LCMV) directed by the human RNA polymerases I and II, respectively, within the same plasmid. This approach reduces by half the number of vectors required for arenavirus rescue, which could facilitate virus rescue in cell lines approved for human vaccine production but that cannot be transfected at high efficiencies. We have shown the feasibility of this approach by rescuing both the Old World prototypic arenavirus LCMV and the live-attenuated vaccine Candid#1 strain of the New World arenavirus Junín. Moreover, we show the feasibility of using these novel strategies for efficient rescue of recombinant tri-segmented both LCMV and Candid#1.
Figures
Similar articles
-
Development of live-attenuated arenavirus vaccines based on codon deoptimization.J Virol. 2015 Apr;89(7):3523-33. doi: 10.1128/JVI.03401-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589652 Free PMC article.
-
Generation of recombinant arenavirus for vaccine development in FDA-approved Vero cells.J Vis Exp. 2013 Aug 1;(78):50662. doi: 10.3791/50662. J Vis Exp. 2013. PMID: 23928556 Free PMC article.
-
Reverse Genetics Approaches to Control Arenavirus.Methods Mol Biol. 2016;1403:313-51. doi: 10.1007/978-1-4939-3387-7_17. Methods Mol Biol. 2016. PMID: 27076139 Free PMC article.
-
Novel strategies for development of hemorrhagic fever arenavirus live-attenuated vaccines.Expert Rev Vaccines. 2016 Sep;15(9):1113-21. doi: 10.1080/14760584.2016.1182024. Epub 2016 May 13. Expert Rev Vaccines. 2016. PMID: 27118328 Free PMC article. Review.
-
Reverse genetics approaches to combat pathogenic arenaviruses.Antiviral Res. 2008 Dec;80(3):239-50. doi: 10.1016/j.antiviral.2008.08.002. Epub 2008 Sep 7. Antiviral Res. 2008. PMID: 18782590 Free PMC article. Review.
Cited by
-
Reverse Genetics Approaches for the Development of Influenza Vaccines.Int J Mol Sci. 2016 Dec 22;18(1):20. doi: 10.3390/ijms18010020. Int J Mol Sci. 2016. PMID: 28025504 Free PMC article. Review.
-
Plasmid-Based Lassa Virus Reverse Genetics.Methods Mol Biol. 2024;2733:115-131. doi: 10.1007/978-1-0716-3533-9_8. Methods Mol Biol. 2024. PMID: 38064030
-
Interferon-Induced Protein 44 Interacts with Cellular FK506-Binding Protein 5, Negatively Regulates Host Antiviral Responses, and Supports Virus Replication.mBio. 2019 Aug 27;10(4):e01839-19. doi: 10.1128/mBio.01839-19. mBio. 2019. PMID: 31455651 Free PMC article.
-
Rapid Generation of Attenuated Infectious Bursal Disease Virus from Dual-Promoter Plasmids by Reduction of Viral Ribonucleoprotein Activity.J Virol. 2020 Mar 17;94(7):e01569-19. doi: 10.1128/JVI.01569-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915284 Free PMC article.
-
Inhibition of arenavirus by A3, a pyrimidine biosynthesis inhibitor.J Virol. 2014 Jan;88(2):878-89. doi: 10.1128/JVI.02275-13. Epub 2013 Nov 6. J Virol. 2014. PMID: 24198417 Free PMC article.
References
-
- Albariño C. G., Bird B. H., Chakrabarti A. K., Dodd K. A., White D. M., Bergeron E., Shrivastava-Ranjan P., Nichol S. T. (2011b). Reverse genetics generation of chimeric infectious Junin/Lassa virus is dependent on interaction of homologous glycoprotein stable signal peptide and G2 cytoplasmic domains. J Virol 85, 112–122 10.1128/JVI.01837-10 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

