The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility
- PMID: 23364267
- PMCID: PMC3651636
- DOI: 10.1152/ajpcell.00383.2012
The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility
Abstract
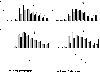
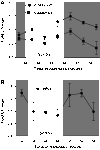
The molecular clock mechanism underlies circadian rhythms and is defined by a transcription-translation feedback loop. Bmal1 encodes a core molecular clock transcription factor. Germline Bmal1 knockout mice show a loss of circadian variation in heart rate and blood pressure, and they develop dilated cardiomyopathy. We tested the role of the molecular clock in adult cardiomyocytes by generating mice that allow for the inducible cardiomyocyte-specific deletion of Bmal1 (iCSΔBmal1). ECG telemetry showed that cardiomyocyte-specific deletion of Bmal1 (iCSΔBmal1(-/-)) in adult mice slowed heart rate, prolonged RR and QRS intervals, and increased episodes of arrhythmia. Moreover, isolated iCSΔBmal1(-/-) hearts were more susceptible to arrhythmia during electromechanical stimulation. Examination of candidate cardiac ion channel genes showed that Scn5a, which encodes the principle cardiac voltage-gated Na(+) channel (Na(V)1.5), was circadianly expressed in control mouse and rat hearts but not in iCSΔBmal1(-/-) hearts. In vitro studies confirmed circadian expression of a human Scn5a promoter-luciferase reporter construct and determined that overexpression of clock factors transactivated the Scn5a promoter. Loss of Scn5a circadian expression in iCSΔBmal1(-/-) hearts was associated with decreased levels of Na(V)1.5 and Na(+) current in ventricular myocytes. We conclude that disruption of the molecular clock in the adult heart slows heart rate, increases arrhythmias, and decreases the functional expression of Scn5a. These findings suggest a potential link between environmental factors that alter the cardiomyocyte molecular clock and factors that influence arrhythmia susceptibility in humans.
Keywords: Na+ current; Scn5a; cardiac excitability; circadian; heart; ion channels.
Figures



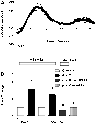

Comment in
-
Minding the gaps that link intrinsic circadian clock within the heart to its intrinsic ultradian pacemaker clocks. Focus on "The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility".Am J Physiol Cell Physiol. 2013 May 15;304(10):C941-4. doi: 10.1152/ajpcell.00072.2013. Epub 2013 Mar 13. Am J Physiol Cell Physiol. 2013. PMID: 23485714 Free PMC article. No abstract available.
References
-
- CAST II Investigators Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. The Cardiac Arrhythmia Suppression Trial II Investigators. N Engl J Med 327: 227–233, 1992 - PubMed
-
- CAST Investigators Preliminary report: effect of encainide, and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 321: 406–412, 1989 - PubMed
-
- Abriel H. Roles and regulation of the cardiac sodium channel Nav1.5: recent insights from experimental studies. Cardiovasc Res 76: 381–389, 2007 - PubMed
-
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540–550, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

