The hematopoietic stem cell regulatory gene latexin has tumor-suppressive properties in malignant melanoma
- PMID: 23364479
- PMCID: PMC3683103
- DOI: 10.1038/jid.2013.48
The hematopoietic stem cell regulatory gene latexin has tumor-suppressive properties in malignant melanoma
Abstract
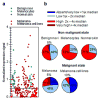
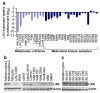
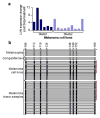
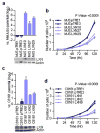
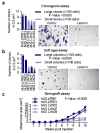
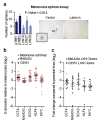
Despite recent advancements in therapy, melanoma remains a highly lethal skin cancer. A better understanding of the genetic and epigenetic changes responsible for melanoma formation and progression could result in the development of more effective treatments. Advanced melanomas are known to exhibit widespread promoter region CpG island methylation leading to the inactivation of key tumor suppressor genes. Meta-analyses of relevant microarray data sets revealed the hematopoietic stem cell regulator gene latexin (LXN) to be commonly downregulated in approximately 50% of melanomas. The CpG island in the promoter region of LXN was almost universally hypermethylated in melanoma cell lines and tumors, and treatment of the cell lines with the demethylating drug 5-aza-2'-deoxycytidine resulted in increased LXN expression. In this paper, we demonstrate that the exogenous expression of LXN in melanoma cell lines results in a significant inhibition of tumor cell proliferation. In addition, we show that the increased expression of LXN in these lines correlates with reduction in the expression levels of stem cell transcription factors OCT4, NANOG, SOX2, KLF4, and MYCN, indicating that LXN may exert its tumor-suppressive function by altering the stem cell-like properties of melanoma cells.
Conflict of interest statement
The authors state no conflict of interest
Figures
Similar articles
-
Latexin expression is downregulated in human gastric carcinomas and exhibits tumor suppressor potential.BMC Cancer. 2011 Apr 6;11:121. doi: 10.1186/1471-2407-11-121. BMC Cancer. 2011. PMID: 21466706 Free PMC article.
-
Epigenetic silencing of novel tumor suppressors in malignant melanoma.Cancer Res. 2006 Dec 1;66(23):11187-93. doi: 10.1158/0008-5472.CAN-06-1274. Cancer Res. 2006. PMID: 17145863
-
Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma.Cancer Res. 2003 Apr 1;63(7):1639-43. Cancer Res. 2003. PMID: 12670917
-
Latexin inhibits the proliferation of CD133+ miapaca-2 pancreatic cancer stem-like cells.World J Surg Oncol. 2014 Dec 30;12:404. doi: 10.1186/1477-7819-12-404. World J Surg Oncol. 2014. PMID: 25551472 Free PMC article.
-
Dipeptidyl peptidase IV (DPPIV), a candidate tumor suppressor gene in melanomas is silenced by promoter methylation.Front Biosci. 2008 Jan 1;13:2435-43. doi: 10.2741/2856. Front Biosci. 2008. PMID: 17981724 Review.
Cited by
-
Latexin Inactivation Enhances Survival and Long-Term Engraftment of Hematopoietic Stem Cells and Expands the Entire Hematopoietic System in Mice.Stem Cell Reports. 2017 Apr 11;8(4):991-1004. doi: 10.1016/j.stemcr.2017.02.009. Epub 2017 Mar 16. Stem Cell Reports. 2017. PMID: 28330618 Free PMC article.
-
Bone Microenvironment Changes in Latexin Expression Promote Chemoresistance.Mol Cancer Res. 2017 Apr;15(4):457-466. doi: 10.1158/1541-7786.MCR-16-0392. Epub 2017 Jan 13. Mol Cancer Res. 2017. PMID: 28087740 Free PMC article.
-
Cancer metastasis - tricks of the trade.Bosn J Basic Med Sci. 2017 Aug 20;17(3):172-182. doi: 10.17305/bjbms.2017.1908. Bosn J Basic Med Sci. 2017. PMID: 28278128 Free PMC article. Review.
-
Aberrant DNA methylation in melanoma: biomarker and therapeutic opportunities.Clin Epigenetics. 2017 Apr 4;9:34. doi: 10.1186/s13148-017-0332-8. eCollection 2017. Clin Epigenetics. 2017. PMID: 28396701 Free PMC article. Review.
-
CEACAM1 promotes melanoma metastasis and is involved in the regulation of the EMT associated gene network in melanoma cells.Sci Rep. 2018 Aug 8;8(1):11893. doi: 10.1038/s41598-018-30338-4. Sci Rep. 2018. PMID: 30089785 Free PMC article.
References
-
- Aagaard A, Listwan P, Cowieson N, Huber T, Ravasi T, Wells CA, et al. An inflammatory role for the mammalian carboxypeptidase inhibitor latexin: relationship to cystatins and the tumor suppressor TIG1. Structure. 2005;13:309–17. - PubMed
-
- Aitken J, Welch J, Duffy D, Milligan A, Green A, Martin N, et al. CDKN2A variants in a population-based sample of Queensland families with melanoma. Journal of the National Cancer Institute. 1999;91:446–52. - PubMed
-
- Arimatsu Y. Latexin: a molecular marker for regional specification in the neocortex. Neuroscience research. 1994;20:131–5. - PubMed
-
- Bai WZ, Ishida M, Arimatsu Y. Chemically defined feedback connections from infragranular layers of sensory association cortices in the rat. Neuroscience. 2004;123:257–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

