Oxidative folding in the mitochondrial intermembrane space in human health and disease
- PMID: 23364613
- PMCID: PMC3588022
- DOI: 10.3390/ijms14022916
Oxidative folding in the mitochondrial intermembrane space in human health and disease
Abstract
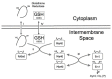
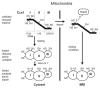
Oxidative folding in the mitochondrial intermembrane space (IMS) is a key cellular event associated with the folding and import of a large and still undetermined number of proteins. This process is catalyzed by an oxidoreductase, Mia40 that is able to recognize substrates with apparently little or no homology. Following substrate oxidation, Mia40 is reduced and must be reoxidized by Erv1/Alr1 that consequently transfers the electrons to the mitochondrial respiratory chain. Although our understanding of the physiological relevance of this process is still limited, an increasing number of pathologies are being associated with the impairment of this pathway; especially because oxidative folding is fundamental for several of the proteins involved in defense against oxidative stress. Here we review these aspects and discuss recent findings suggesting that oxidative folding in the IMS is modulated by the redox state of the cell.
Figures
References
-
- Schmidt O., Pfanner N., Meisinger C. Mitochondrial protein import: From proteomics to functional mechanisms. Nat. Rev. Mol. Cell. Biol. 2010;11:655–667. - PubMed
-
- Baker K.P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991;349:205–208. - PubMed
-
- Dessi P., Whelan J. Temporal regulation of in vitro import of precursor proteins into tobacco mitochondria. FEBS. Lett. 1997;415:173–178. - PubMed
-
- Dudley P., Wood C.K., Pratt J.R., Moore A.L. Developmental regulation of the plant mitochondrial matrix located Hsp70 chaperone and its role in protein import. FEBS Lett. 1997;417:321–324. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

