Iodinin (1,6-dihydroxyphenazine 5,10-dioxide) from Streptosporangium sp. induces apoptosis selectively in myeloid leukemia cell lines and patient cells
- PMID: 23364682
- PMCID: PMC3640383
- DOI: 10.3390/md11020332
Iodinin (1,6-dihydroxyphenazine 5,10-dioxide) from Streptosporangium sp. induces apoptosis selectively in myeloid leukemia cell lines and patient cells
Abstract
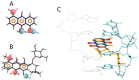
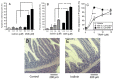
Despite recent improvement in therapy, acute myeloid leukemia (AML) is still associated with high lethality. In the presented study, we analyzed the bioactive compound iodinin (1,6-dihydroxyphenazine 5,10-dioxide) from a marine actinomycetes bacterium for the ability to induce cell death in a range of cell types. Iodinin showed selective toxicity to AML and acute promyelocytic (APL) leukemia cells, with EC50 values for cell death up to 40 times lower for leukemia cells when compared with normal cells. Iodinin also successfully induced cell death in patient-derived leukemia cells or cell lines with features associated with poor prognostic such as FLT3 internal tandem duplications or mutated/deficient p53. The cell death had typical apoptotic morphology, and activation of apoptotic signaling proteins like caspase-3. Molecular modeling suggested that iodinin could intercalate between bases in the DNA in a way similar to the anti-cancer drug daunorubicin (DNR), causing DNA-strand breaks. Iodinin induced apoptosis in several therapy-resistant AML-patient blasts, but to a low degree in peripheral blood leukocytes, and in contrast to DNR, not in rat cardiomyoblasts. The low activity towards normal cell types that are usually affected by anti-leukemia therapy suggests that iodinin and related compounds represent promising structures in the development of anti-cancer therapy.
Figures





References
-
- Fenaux P., Chastang C., Chevret S., Sanz M., Dombret H., Archimbaud E., Fey M., Rayon C., Huguet F., Sotto J.J., et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. Blood. 1999;94:1192–1200. - PubMed
-
- Mehta A., Hoffbrand V. Haematology at a Glance. 3rd. Wiley-Blackwell; Oxford, UK: 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

