Enhanced aggregation of androgen receptor in induced pluripotent stem cell-derived neurons from spinal and bulbar muscular atrophy
- PMID: 23364790
- PMCID: PMC3605623
- DOI: 10.1074/jbc.M112.408211
Enhanced aggregation of androgen receptor in induced pluripotent stem cell-derived neurons from spinal and bulbar muscular atrophy
Abstract
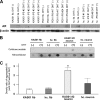
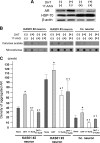
Spinal and bulbar muscular atrophy (SBMA) is an X-linked motor neuron disease caused by a CAG repeat expansion in the androgen receptor (AR) gene. Ligand-dependent nuclear accumulation of mutant AR protein is a critical characteristic of the pathogenesis of SBMA. SBMA has been modeled in AR-overexpressing animals, but precisely how the polyglutamine (polyQ) expansion leads to neurodegeneration is unclear. Induced pluripotent stem cells (iPSCs) are a new technology that can be used to model human diseases, study pathogenic mechanisms, and develop novel drugs. We established SBMA patient-derived iPSCs, investigated their cellular biochemical characteristics, and found that SBMA-iPSCs can differentiate into motor neurons. The CAG repeat numbers in the AR gene of SBMA-iPSCs and also in the atrophin-1 gene of iPSCs derived from another polyQ disease, dentato-rubro-pallido-luysian atrophy (DRPLA), remain unchanged during reprogramming, long term passage, and differentiation, indicating that polyQ disease-associated CAG repeats are stable during maintenance of iPSCs. The level of AR expression is up-regulated by neuronal differentiation and treatment with the AR ligand dihydrotestosterone. Filter retardation assays indicated that aggregation of ARs following dihydrotestosterone treatment in neurons derived from SBMA-iPSCs increases significantly compared with neurological control iPSCs, easily recapitulating the pathological feature of mutant ARs in SBMA-iPSCs. This phenomenon was not observed in iPSCs and fibroblasts, thereby showing the neuron-dominant phenotype of this disease. Furthermore, the HSP90 inhibitor 17-allylaminogeldanamycin sharply decreased the level of aggregated AR in neurons derived from SBMA-iPSCs, indicating a potential for discovery and validation of candidate drugs. We found that SBMA-iPSCs possess disease-specific biochemical features and could thus open new avenues of research into not only SBMA, but also other polyglutamine diseases.
Figures

Similar articles
-
Mutant androgen receptor induces neurite loss and senescence independently of ARE binding in a neuronal model of SBMA.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2321408121. doi: 10.1073/pnas.2321408121. Epub 2024 Jul 8. Proc Natl Acad Sci U S A. 2024. PMID: 38976730 Free PMC article.
-
Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA).Prog Neurobiol. 2012 Dec;99(3):246-56. doi: 10.1016/j.pneurobio.2012.05.007. Epub 2012 May 15. Prog Neurobiol. 2012. PMID: 22609045 Review.
-
Modulation of Hsp90 function in neurodegenerative disorders: a molecular-targeted therapy against disease-causing protein.J Mol Med (Berl). 2006 Aug;84(8):635-46. doi: 10.1007/s00109-006-0066-0. Epub 2006 Jun 2. J Mol Med (Berl). 2006. PMID: 16741751 Review.
-
Pathogenesis and molecular targeted therapy of spinal and bulbar muscular atrophy.Neuropathol Appl Neurobiol. 2007 Apr;33(2):135-51. doi: 10.1111/j.1365-2990.2007.00830.x. Neuropathol Appl Neurobiol. 2007. PMID: 17359355 Review.
-
Human adipose-derived mesenchymal stem cells as a new model of spinal and bulbar muscular atrophy.PLoS One. 2014 Nov 13;9(11):e112746. doi: 10.1371/journal.pone.0112746. eCollection 2014. PLoS One. 2014. PMID: 25392924 Free PMC article.
Cited by
-
Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons.Acta Neuropathol. 2013 Sep;126(3):385-99. doi: 10.1007/s00401-013-1149-y. Epub 2013 Jul 9. Acta Neuropathol. 2013. PMID: 23836290 Free PMC article.
-
Exploring Motor Neuron Diseases Using iPSC Platforms.Stem Cells. 2022 Mar 3;40(1):2-13. doi: 10.1093/stmcls/sxab006. Stem Cells. 2022. PMID: 35511862 Free PMC article.
-
Advances in Modeling Polyglutamine Diseases Using Genome Editing Tools.Cells. 2022 Feb 2;11(3):517. doi: 10.3390/cells11030517. Cells. 2022. PMID: 35159326 Free PMC article. Review.
-
Induced pluripotent stem cells (iPSCs) as model to study inherited defects of neurotransmission in inborn errors of metabolism.J Inherit Metab Dis. 2018 Nov;41(6):1103-1116. doi: 10.1007/s10545-018-0225-9. Epub 2018 Jul 6. J Inherit Metab Dis. 2018. PMID: 29980968 Review.
-
Unveiling synapse pathology in spinal bulbar muscular atrophy by genome-wide transcriptome analysis of purified motor neurons derived from disease specific iPSCs.Mol Brain. 2020 Feb 19;13(1):18. doi: 10.1186/s13041-020-0561-1. Mol Brain. 2020. PMID: 32070397 Free PMC article.
References
-
- Kennedy W. R., Alter M., Sung J. H. (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 18, 671–680 - PubMed
-
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 - PubMed
-
- Brooks B. P., Fischbeck K. H. (1995) Spinal and bulbar muscular atrophy. A trinucleotide repeat expansion neurodegenerative disease. Trends Neurosci., 18, 459–461 - PubMed
-
- Fischbeck K. H. (1997) Kennedy disease. J. Inher. Metab. Dis. 20, 152–158 - PubMed
-
- Fischbeck K. H. (2001) PolyQ expansion neurodegenerative disease. Brain Res. Bull. 56, 161–163 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

