Myogenin recruits the histone chaperone facilitates chromatin transcription (FACT) to promote nucleosome disassembly at muscle-specific genes
- PMID: 23364797
- PMCID: PMC3597808
- DOI: 10.1074/jbc.M112.426718
Myogenin recruits the histone chaperone facilitates chromatin transcription (FACT) to promote nucleosome disassembly at muscle-specific genes
Abstract
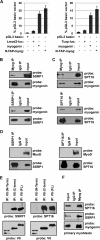
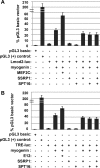
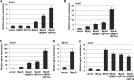
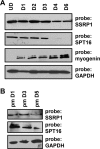
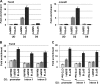
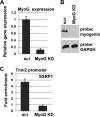
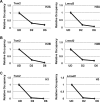
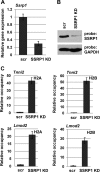
Facilitates chromatin transcription (FACT) functions to reorganize nucleosomes by acting as a histone chaperone that destabilizes and restores nucleosomal structure. The FACT complex is composed of two subunits: SSRP1 and SPT16. We have discovered that myogenin interacts with the FACT complex. Transfection of FACT subunits with myogenin is highly stimulatory for endogenous muscle gene expression in 10T1/2 cells. We have also found that FACT subunits do not associate with differentiation-specific genes while C2C12 cells are proliferating but are recruited to muscle-specific genes as differentiation initiates and then dissociate as differentiation proceeds. The recruitment is dependent on myogenin, as knockdowns of myogenin show no recruitment of the FACT complex. These data suggest that FACT is involved in the early steps of gene activation through its histone chaperone activities that serve to open the chromatin structure and facilitate transcription. Consistent with this hypothesis, we find that nucleosomes are depleted at muscle-specific promoters upon differentiation and that this activity is dependent on the presence of FACT. Our results show that the FACT complex promotes myogenin-dependent transcription and suggest that FACT plays an important role in the establishment of the appropriate transcription profile in a differentiated muscle cell.
Figures
References
-
- Berkes C. A., Tapscott S. J. (2005) MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16, 585–595 - PubMed
-
- Molkentin J. D., Black B. L., Martin J. F., Olson E. N. (1995) Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83, 1125–1136 - PubMed
-
- Wang D. Z., Valdez M. R., McAnally J., Richardson J., Olson E. N. (2001) The Mef2c gene is a direct transcriptional target of myogenic bHLH and MEF2 proteins during skeletal muscle development. Development 128, 4623–4633 - PubMed
-
- Rudnicki M. A., Schnegelsberg P. N., Stead R. H., Braun T., Arnold H. H., Jaenisch R. (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351–1359 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

