Optimizing the 6-min walk test as a measure of exercise capacity in COPD
- PMID: 23364913
- PMCID: PMC3515028
- DOI: 10.1378/chest.11-2702
Optimizing the 6-min walk test as a measure of exercise capacity in COPD
Abstract
Background: It is uncertain whether the effort and expense of performing a second walk for the 6-min walk test improves test performance. Hence, we attempted to quantify the improvement in 6-min walk distance if an additional walk were to be performed.
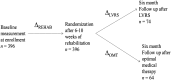
Methods: We studied patients consecutively enrolled into the National Emphysema Treatment Trial who prior to randomization and after 6 to 10 weeks of pulmonary rehabilitation performed two 6-min walks on consecutive days (N = 396). Patients also performed two 6-min walks at 6-month follow-up after randomization to lung volume reduction surgery (n = 74) or optimal medical therapy (n = 64). We compared change in the first walk distance to change in the second, average-of-two, and best-of-two walk distances.
Results: Compared with the change in the first walk distance, change in the average-of-two and best-of-two walk distances had better validity and precision. Specifically, 6 months after randomization to lung volume reduction surgery, changes in the average-of-two (r = 0.66 vs r = 0.58, P = .01) and best-of-two walk distances (r = 0.67 vs r = 0.58, P = .04) better correlated with the change in maximal exercise capacity (ie, better validity). Additionally, the variance of change was 14% to 25% less for the average-of-two walk distances and 14% to 33% less for the best-of-two walk distances than the variance of change in the single walk distance, indicating better precision.
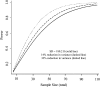
Conclusions: Adding a second walk to the 6-min walk test significantly improves its performance in measuring response to a therapeutic intervention, improves the validity of COPD clinical trials, and would result in a 14% to 33% reduction in sample size requirements. Hence, it should be strongly considered by clinicians and researchers as an outcome measure for therapeutic interventions in patients with COPD.
Figures

References
-
- Salzman SH. The 6-min walk test: clinical and research role, technique, coding, and reimbursement. Chest. 2009;135(5):1345-1352 - PubMed
-
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117 - PubMed
-
- National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073 - PubMed
-
- Patel SA, Sciurba FC. Emerging concepts in outcome assessment for COPD clinical trials. Semin Respir Crit Care Med. 2005;26(2):253-262 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01HR76113/HR/NHLBI NIH HHS/United States
- N01HR76104/HR/NHLBI NIH HHS/United States
- N01HR76105/HR/NHLBI NIH HHS/United States
- N01 HR076101/HL/NHLBI NIH HHS/United States
- N01HR76108/HR/NHLBI NIH HHS/United States
- N01HR76117/HR/NHLBI NIH HHS/United States
- N01HR76109/HR/NHLBI NIH HHS/United States
- N01HR76116/HR/NHLBI NIH HHS/United States
- N01HR76112/HR/NHLBI NIH HHS/United States
- N01HR76107/HR/NHLBI NIH HHS/United States
- N01HR76102/HR/NHLBI NIH HHS/United States
- N01HR76118/HR/NHLBI NIH HHS/United States
- N01HR76115/HR/NHLBI NIH HHS/United States
- N01HR76111/HR/NHLBI NIH HHS/United States
- N01HR76114/HR/NHLBI NIH HHS/United States
- N01HR76103/HR/NHLBI NIH HHS/United States
- P50HL084948/HL/NHLBI NIH HHS/United States
- N01HR76110/HR/NHLBI NIH HHS/United States
- P50 HL084948/HL/NHLBI NIH HHS/United States
- N01 HR076119/HL/NHLBI NIH HHS/United States
- N01HR76106/HR/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

