Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death
- PMID: 23364915
- PMCID: PMC3663913
- DOI: 10.1002/ijc.28070
Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death
Abstract
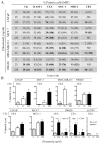
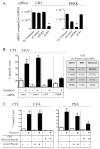
Certain chemotherapeutic regimens trigger cancer cell death while inducing dendritic cell maturation and subsequent immune responses. However, chemotherapy-induced immunogenic cell death (ICD) has thus far been restricted to select agents. In contrast, several chemotherapeutic drugs modulate antitumor immune responses, despite not inducing classic ICD. In addition, in many cases tumor cells do not die after treatment. Here, using docetaxel, one of the most widely used cancer chemotherapeutic agents, as a model, we examined phenotypic and functional consequences of tumor cells that do not die from ICD. Docetaxel treatment of tumor cells did not induce ATP or high-mobility group box 1 (HMGB1) secretion, or cell death. However, calreticulin (CRT) exposure was observed in all cell lines examined after chemotherapy treatment. Killing by carcinoembryonic antigen (CEA), MUC-1, or PSA-specific CD8(+) CTLs was significantly enhanced after docetaxel treatment. This killing was associated with increases in components of antigen-processing machinery, and mediated largely by CRT membrane translocation, as determined by functional knockdown of CRT, PERK, or CRT-blocking peptide. A docetaxel-resistant cell line was selected (MDR-1(+), CD133(+)) by continuous exposure to docetaxel. These cells, while resistant to direct cytostatic effects of docetaxel, were not resistant to the chemomodulatory effects that resulted in enhancement of CTL killing. Here, we provide an operational definition of "immunogenic modulation," where exposure of tumor cells to nonlethal/sublethal doses of chemotherapy alters tumor phenotype to render the tumor more sensitive to CTL killing. These observations are distinct and complementary to ICD and highlight a mechanism whereby chemotherapy can be used in combination with immunotherapy.
Copyright © 2013 UICC.
Figures




Similar articles
-
Docetaxel enhances CD3+ CD56+ cytokine-induced killer cells-mediated killing through inducing tumor cells phenotype modulation.Biomed Pharmacother. 2015 Feb;69:18-23. doi: 10.1016/j.biopha.2014.10.026. Epub 2014 Nov 6. Biomed Pharmacother. 2015. PMID: 25661332
-
Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing.Oncotarget. 2014 Jan 30;5(2):403-16. doi: 10.18632/oncotarget.1719. Oncotarget. 2014. PMID: 24480782 Free PMC article.
-
Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation.Oncotarget. 2016 Dec 27;7(52):86937-86947. doi: 10.18632/oncotarget.13520. Oncotarget. 2016. PMID: 27893426 Free PMC article.
-
Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies.Cancer Lett. 2018 Dec 1;438:17-23. doi: 10.1016/j.canlet.2018.08.028. Epub 2018 Sep 11. Cancer Lett. 2018. PMID: 30217563 Review.
-
Molecular determinants of immunogenic cell death: surface exposure of calreticulin makes the difference.J Mol Med (Berl). 2007 Oct;85(10):1069-76. doi: 10.1007/s00109-007-0214-1. Epub 2007 May 22. J Mol Med (Berl). 2007. PMID: 17891368 Review.
Cited by
-
Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer.Oncotarget. 2015 Jul 20;6(20):18192-205. doi: 10.18632/oncotarget.4145. Oncotarget. 2015. PMID: 26078335 Free PMC article. Clinical Trial.
-
A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy.Nanomicro Lett. 2020 Jul 5;12(1):142. doi: 10.1007/s40820-020-00482-6. Nanomicro Lett. 2020. PMID: 34138136 Free PMC article. Review.
-
Effectiveness and safety of combined therapy versus monotherapy based on immune checkpoint inhibitors and/or targeted drugs as salvage treatment for advanced urothelial carcinoma: a systematic review and meta-analysis.Transl Cancer Res. 2021 May;10(5):2091-2107. doi: 10.21037/tcr-20-3354. Transl Cancer Res. 2021. PMID: 35116530 Free PMC article.
-
SS1P Immunotoxin Induces Markers of Immunogenic Cell Death and Enhances the Effect of the CTLA-4 Blockade in AE17M Mouse Mesothelioma Tumors.Toxins (Basel). 2018 Nov 14;10(11):470. doi: 10.3390/toxins10110470. Toxins (Basel). 2018. PMID: 30441807 Free PMC article.
-
A novel function of API5 (apoptosis inhibitor 5), TLR4-dependent activation of antigen presenting cells.Oncoimmunology. 2018 Aug 15;7(10):e1472187. doi: 10.1080/2162402X.2018.1472187. eCollection 2018. Oncoimmunology. 2018. PMID: 30288341 Free PMC article.
References
-
- Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. - PubMed
-
- Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–64. - PubMed
-
- Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66:185–94. - PubMed
-
- Ogino T, Wang X, Ferrone S. Modified flow cytometry and cell-ELISA methodology to detect HLA class I antigen processing machinery components in cytoplasm and endoplasmic reticulum. J Immunol Methods. 2003;278:33–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

