Intrathecal lamotrigine attenuates mechanical allodynia and suppresses microglial and astrocytic activation in a rat model of spinal nerve ligation
- PMID: 23364963
- PMCID: PMC3575973
- DOI: 10.3349/ymj.2013.54.2.321
Intrathecal lamotrigine attenuates mechanical allodynia and suppresses microglial and astrocytic activation in a rat model of spinal nerve ligation
Abstract
Purpose: Lamotrigine, a novel anticonvulsant, is a sodium channel blocker that is efficacious in certain forms of neuropathic pain. Recently, microglial and astrocytic activation has been implicated in the development of nerve injury-induced neuropathic pain. We have assessed the effects of continuous intrathecal administration of lamotrigine on the development of neuropathic pain and glial activation induced by L5/6 spinal-nerve ligation in rats.
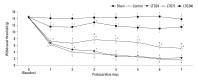
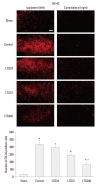
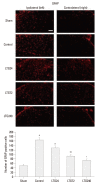
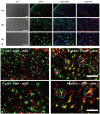
Materials and methods: Following left L5/6 spinal nerve ligation (SNL), Sprague-Dawley male rats were intrathecally administered lamotrigine (24, 72, or 240 μg/day) or saline continuously for 7 days. Mechanical allodynia of the left hind paw to von Frey filament stimuli was determined before surgery (baseline) and once daily for 7 days postoperatively. On day 7, spinal activation of microglia and astrocytes was evaluated immunohistochemically, using antibodies to the microglial marker OX-42 and the astrocyte marker glial fibrillary acidic protein (GFAP).
Results: Spinal-nerve ligation induced mechanical allodynia in saline-treated rats, with OX-42 and GFAP immunoreactivity being significantly increased on the ipsilateral side of the spinal cord. Continuously administered intrathecal lamotrigine (240 μg/day) prevented the development of mechanical allodynia, and lower dose of lamotrigine (72 μg/day) ameliorated allodynia. Intrathecal lamotrigine (72 and 240 μg/day) inhibited nerve ligation-induced microglial and astrocytic activation, as evidenced by reduced numbers of cells positive for OX-42 and GFAP.
Conclusion: Continuously administered intrathecal lamotrigine blocked the development of mechanical allodynia induced by SNL with suppression of microglial and astrocytic activation. Continuous intrathecal administration of lamotrigine may be a promising therapeutic intervention to prevent neuropathy.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


Similar articles
-
Activation of astrocytes in the spinal cord contributes to the development of bilateral allodynia after peripheral nerve injury in rats.Brain Res. 2010 Dec 2;1363:72-80. doi: 10.1016/j.brainres.2010.09.105. Epub 2010 Oct 25. Brain Res. 2010. PMID: 20932955
-
Chronic intrathecal infusion of minocycline prevents the development of spinal-nerve ligation-induced pain in rats.Reg Anesth Pain Med. 2007 May-Jun;32(3):209-16. doi: 10.1016/j.rapm.2007.01.005. Reg Anesth Pain Med. 2007. PMID: 17543815
-
Inhibition of spinal microglia and astrocytes contributes to the anti-allodynic effect of electroacupuncture in neuropathic pain induced by spinal nerve ligation.Acupunct Med. 2016 Feb;34(1):40-7. doi: 10.1136/acupmed-2015-010773. Epub 2015 Jul 15. Acupunct Med. 2016. PMID: 26177687
-
Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation.J Neuroinflammation. 2018 Dec 20;15(1):349. doi: 10.1186/s12974-018-1378-z. J Neuroinflammation. 2018. PMID: 30572902 Free PMC article.
-
Goshajinkigan attenuates paclitaxel-induced neuropathic pain via cortical astrocytes.Pharmacol Res Perspect. 2021 Dec;9(6):e00850. doi: 10.1002/prp2.850. Pharmacol Res Perspect. 2021. PMID: 34676996 Free PMC article. Review.
Cited by
-
Anti-allodynic effect of intrathecal processed Aconitum jaluense is associated with the inhibition of microglial activation and P2X7 receptor expression in spinal cord.BMC Complement Altern Med. 2016 Jul 13;16:214. doi: 10.1186/s12906-016-1201-2. BMC Complement Altern Med. 2016. PMID: 27411500 Free PMC article.
-
Effects of Lamotrigine and Topiramate on Glial Properties in an Astrocyte-Microglia Co-Culture Model of Inflammation.Int J Neuropsychopharmacol. 2022 Mar 17;25(3):185-196. doi: 10.1093/ijnp/pyab080. Int J Neuropsychopharmacol. 2022. PMID: 34791253 Free PMC article.
-
ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain.Int J Mol Sci. 2020 Jul 27;21(15):5330. doi: 10.3390/ijms21155330. Int J Mol Sci. 2020. PMID: 32727084 Free PMC article. Review.
References
-
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. - PMC - PubMed
-
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. - PubMed
-
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. - PubMed
-
- Lees G, Leach MJ. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res. 1993;612:190–199. - PubMed
-
- McCleane GJ. Lamotrigine in the management of neuropathic pain: a review of the literature. Clin J Pain. 2000;16:321–326. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

