Rapamycin inhibits transforming growth factor β1-induced fibrogenesis in primary human lung fibroblasts
- PMID: 23364979
- PMCID: PMC3576000
- DOI: 10.3349/ymj.2013.54.2.437
Rapamycin inhibits transforming growth factor β1-induced fibrogenesis in primary human lung fibroblasts
Abstract
Purpose: The present study was designed to determine whether rapamycin could inhibit transforming growth factor β1 (TGF-β1)-induced fibrogenesis in primary lung fibroblasts, and whether the effect of inhibition would occur through the mammalian target of rapamycin (mTOR) and its downstream p70S6K pathway.
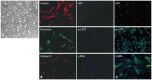
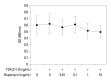
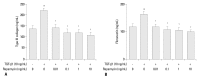
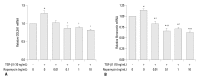
Materials and methods: Primary normal human lung fibroblasts were obtained from histological normal lung tissue of 3 patients with primary spontaneous pneumothorax. Growth arrested, synchronized fibroblasts were treated with TGF-β1 (10 ng/mL) and different concentrations of rapamycin (0.01, 0.1, 1, 10 ng/mL) for 24 h. We assessed m-TOR, p-mTOR, S6K1, p-S6K1 by Western blot analysis, detected type III collagen and fibronectin secreting by ELISA assay, and determined type III collagen and fibronectin mRNA levels by real-time PCR assay.
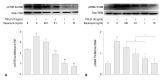
Results: Rapamycin significantly reduced TGF-β1-induced type III collagen and fibronectin levels, as well as type III collagen and fibronectin mRNA levels. Furthermore, we also found that TGF-β1-induced mTOR and p70S6K phosphorylation were significantly down-regulated by rapamycin. The mTOR/p70S6K pathway was activated through the TGF-β1-mediated fibrogenic response in primary human lung fibroblasts.
Conclusion: These results indicate that rapamycin effectively suppresses TGF-β1-induced type III collagen and fibronectin levels in primary human lung fibroblasts partly through the mTOR/p70S6K pathway. Rapamycin has a potential value in the treatment of pulmonary fibrosis.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
Similar articles
-
Isoliquiritigenin inhibits TGF-β1-induced fibrogenesis through activating autophagy via PI3K/AKT/mTOR pathway in MRC-5 cells.Acta Biochim Biophys Sin (Shanghai). 2020 Aug 5;52(8):810-820. doi: 10.1093/abbs/gmaa067. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 32638014
-
Role of PI3K/Akt/mTOR pathway-mediated macrophage autophagy in affecting the phenotype transformation of lung fibroblasts induced by silica dust exposure.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Aug 28;48(8):1152-1162. doi: 10.11817/j.issn.1672-7347.2023.220581. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37875355 Free PMC article. Chinese, English.
-
Effects of mammalian target of rapamycin inhibitors on fibrosis after trabeculectomy.Exp Eye Res. 2021 Feb;203:108421. doi: 10.1016/j.exer.2020.108421. Epub 2020 Dec 24. Exp Eye Res. 2021. PMID: 33359326
-
Hydroxysafflor yellow A inhibits TGF-β1-induced activation of human fetal lung fibroblasts in vitro.J Pharm Pharmacol. 2016 Oct;68(10):1320-30. doi: 10.1111/jphp.12596. Epub 2016 Jul 26. J Pharm Pharmacol. 2016. PMID: 27457091
-
Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review.
Cited by
-
Plumbagin attenuates traumatic tracheal stenosis in rats and inhibits lung fibroblast proliferation and differentiation via TGF-β1/Smad and Akt/mTOR pathways.Bioengineered. 2021 Dec;12(1):4475-4488. doi: 10.1080/21655979.2021.1954580. Bioengineered. 2021. PMID: 34304701 Free PMC article.
-
Rapamycin increases CCN2 expression of lung fibroblasts via phosphoinositide 3-kinase.Lab Invest. 2015 Aug;95(8):846-59. doi: 10.1038/labinvest.2015.68. Epub 2015 Jul 20. Lab Invest. 2015. PMID: 26192087
-
Steady Augmentation of Anti-Osteoarthritic Actions of Rapamycin by Liposome-Encapsulation in Collaboration with Low-Intensity Pulsed Ultrasound.Int J Nanomedicine. 2020 May 28;15:3771-3790. doi: 10.2147/IJN.S252223. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32547027 Free PMC article.
-
Prolonged Scar-in-a-Jar: an in vitro screening tool for anti-fibrotic therapies using biomarkers of extracellular matrix synthesis.Respir Res. 2020 May 7;21(1):108. doi: 10.1186/s12931-020-01369-1. Respir Res. 2020. PMID: 32381012 Free PMC article.
-
Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells.BMC Pulm Med. 2018 Apr 27;18(1):63. doi: 10.1186/s12890-018-0626-4. BMC Pulm Med. 2018. PMID: 29703175 Free PMC article.
References
-
- Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res. 2007;24:819–841. - PubMed
-
- Andersson-Sjöland A, de Alba CG, Nihlberg K, Becerril C, Ramírez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. - PubMed
-
- Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

