Rapid decrease of intact parathyroid hormone could be a predictor of better response to cinacalcet in hemodialysis patients
- PMID: 23364981
- PMCID: PMC3575968
- DOI: 10.3349/ymj.2013.54.2.453
Rapid decrease of intact parathyroid hormone could be a predictor of better response to cinacalcet in hemodialysis patients
Abstract
Purpose: Cinacalcet is effective for treating refractory secondary hyperparathyroidism (SHPT), but little is known about the response rates and clinical factors influencing the response.
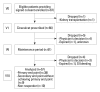
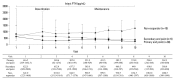
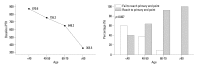
Materials and methods: A prospective, single-arm, multi-center study was performed for 24 weeks. Cinacalcet was administered to patients with intact parathyroid hormone (iPTH) level greater than 300 pg/mL. Cinacalcet was started at a dose of 25 mg daily and titrated until 100 mg to achieve a serum iPTH level<300 pg/mL (primary end point). Early response to cinacalcet was defined as a decrease of iPTH more than 50% within one month.
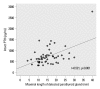
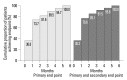
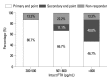
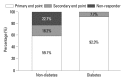
Results: Fifty-seven patients were examined. Based on the magnitude of iPTH decrease, patients were divided into responder (n=47, 82.5%) and non-responder (n=10, 17.5%) groups. Among the responders, 38 achieved the primary end point, whereas 9 patients showed a reduction in serum iPTH of 30% or more, but did not reach the primary end point. Compared to non-responders, responders were significantly older (p=0.026), female (p=0.041), and diabetics (p<0.001). Additionally, early response was observed more frequently in the responders (30/47, 63.8%), of whom the majority (27/30, 90.0%) achieved the primary end point. Multivariate analysis showed that lower baseline iPTH levels [odds ratio (OR) 0.96, 95% confidence interval (CI) 0.93-0.99], the presence of diabetes (OR 46.45, CI 1.92-1125.6) and early response (OR 21.54, CI 2.94-157.7) were significant clinical factors affecting achievement of iPTH target.
Conclusion: Cinacalcet was effective in most hemodialysis patients with refractory SHPT. The presence of an early response was closely associated with the achievement of target levels of iPTH.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

References
-
- Block GA, Zaun D, Smits G, Persky M, Brillhart S, Nieman K, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78:578–589. - PubMed
-
- Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. - PubMed
-
- Cozzolino M, Mazzaferro S, Messa P. New insights into the role of calcium-sensing receptor activation. J Nephrol. 2011;24(Suppl 18):S38–S41. - PubMed
-
- Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005;67:760–771. - PubMed
-
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68:1793–1800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

