The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination
- PMID: 23365095
- PMCID: PMC4572509
- DOI: 10.1093/brain/aws284
The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination
Abstract
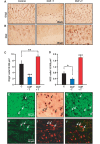
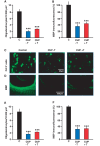
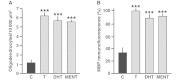
Myelin regeneration is a major therapeutic goal in demyelinating diseases, and the failure to remyelinate rapidly has profound consequences for the health of axons and for brain function. However, there is no efficient treatment for stimulating myelin repair, and current therapies are limited to anti-inflammatory agents. Males are less likely to develop multiple sclerosis than females, but often have a more severe disease course and reach disability milestones at an earlier age than females, and these observations have spurred interest in the potential protective effects of androgens. Here, we demonstrate that testosterone treatment efficiently stimulates the formation of new myelin and reverses myelin damage in chronic demyelinated brain lesions, resulting from the long-term administration of cuprizone, which is toxic for oligodendrocytes. In addition to the strong effect of testosterone on myelin repair, the number of activated astrocytes and microglial cells returned to low control levels, indicating a reduction of neuroinflammatory responses. We also identify the neural androgen receptor as a novel therapeutic target for myelin recovery. After the acute demyelination of cerebellar slices in organotypic culture, the remyelinating actions of testosterone could be mimicked by 5α-dihydrotestosterone, a metabolite that is not converted to oestrogens, and blocked by the androgen receptor antagonist flutamide. Testosterone treatment also failed to promote remyelination after chronic cuprizone-induced demyelination in mice with a non-functional androgen receptor. Importantly, testosterone did not stimulate the formation of new myelin sheaths after specific knockout of the androgen receptor in neurons and macroglial cells. Thus, the neural brain androgen receptor is required for the remyelination effect of testosterone, whereas the presence of the receptor in microglia and in peripheral tissues is not sufficient to enhance remyelination. The potent synthetic testosterone analogue 7α-methyl-19-nortestosterone, which has been developed for long-term male contraception and androgen replacement therapy in hypogonadal males and does not stimulate prostate growth, also efficiently promoted myelin repair. These data establish the efficacy of androgens as remyelinating agents and qualify the brain androgen receptor as a promising drug target for remyelination therapy, thus providing the preclinical rationale for a novel therapeutic use of androgens in males with multiple sclerosis.
Figures





Similar articles
-
Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex.Glia. 2015 Jan;63(1):104-17. doi: 10.1002/glia.22736. Epub 2014 Aug 4. Glia. 2015. PMID: 25092805 Free PMC article.
-
Cuprizone-induced demyelination in the rat cerebral cortex and thyroid hormone effects on cortical remyelination.Exp Neurol. 2012 May;235(1):357-67. doi: 10.1016/j.expneurol.2012.02.018. Epub 2012 Mar 7. Exp Neurol. 2012. PMID: 22421533
-
Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination.Brain. 2013 Jan;136(Pt 1):147-67. doi: 10.1093/brain/aws262. Epub 2012 Dec 24. Brain. 2013. PMID: 23266461
-
Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination.Int J Mol Sci. 2020 Apr 30;21(9):3163. doi: 10.3390/ijms21093163. Int J Mol Sci. 2020. PMID: 32365806 Free PMC article. Review.
-
Remyelinating strategies in multiple sclerosis.Expert Rev Neurother. 2014 Nov;14(11):1315-34. doi: 10.1586/14737175.2014.969241. Expert Rev Neurother. 2014. PMID: 25331418 Review.
Cited by
-
Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience.Int J Mol Sci. 2022 Mar 17;23(6):3269. doi: 10.3390/ijms23063269. Int J Mol Sci. 2022. PMID: 35328690 Free PMC article. Review.
-
Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination.Nat Commun. 2023 Mar 22;14(1):1592. doi: 10.1038/s41467-023-36846-w. Nat Commun. 2023. PMID: 36949062 Free PMC article.
-
A systems medicine approach reveals disordered immune system and lipid metabolism in multiple sclerosis patients.Clin Exp Immunol. 2018 Apr;192(1):18-32. doi: 10.1111/cei.13087. Epub 2018 Jan 25. Clin Exp Immunol. 2018. PMID: 29194580 Free PMC article.
-
Sex differences in the neuronal transcriptome and synaptic mitochondrial function in the cerebral cortex of a multiple sclerosis model.Front Neurol. 2023 Nov 2;14:1268411. doi: 10.3389/fneur.2023.1268411. eCollection 2023. Front Neurol. 2023. PMID: 38020654 Free PMC article.
-
Multiple sclerosis; a disease of reproductive-aged women and the dilemma involving contraceptive methods.J Turk Ger Gynecol Assoc. 2015 Mar 1;16(1):49-53. doi: 10.5152/jtgga.2015.15186. eCollection 2015. J Turk Ger Gynecol Assoc. 2015. PMID: 25788851 Free PMC article. Review.
References
-
- Anderson RA, Wallace AM, Sattar N, Kumar N, Sundaram K. Evidence for tissue selectivity of the synthetic androgen 7 alpha-methyl-19-nortestosterone in hypogonadal men. J Clin Endocrinol Metab. 2003;88:2784–93. - PubMed
-
- Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res. 2004;78:157–66. - PubMed
-
- Blakemore WF, Franklin RJ. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212. - PubMed

